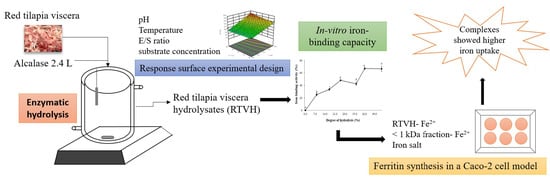
Optimization of the Red Tilapia (Oreochromis spp.) Viscera Hydrolysis for Obtaining Iron-Binding Peptides and Evaluation of In Vitro Iron Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Enzymatic Hydrolysis
2.3.1. Determination of Total Peptide Bonds in the Protein (ht)
2.3.2. Hydrolysis Process
2.3.3. Effect of Substrate Concentration, Substrate Enzyme Ratio, pH, and Temperature on RTV Hydrolysis
2.4. Fractionation of RTV Hydrolysate
2.5. Iron Determination
2.6. In-Vitro Iron-Binding Capacity
2.7. Cytotoxic Assays
2.8. Iron Bioavailability
2.8.1. Cell Culture
2.8.2. Ferritin Assay
2.9. Statistical Analysis
3. Results
3.1. Effect of S, E/S, pH, and Temperature on RTV Hydrolysis
3.2. Effect of DH on Iron-Binding Activity of Hydrolysates
3.3. Fractionation by Molecular Weight of the Hydrolysate with the Highest Iron Chelating Activity
3.4. Bioavailability of Iron
3.4.1. Cytotoxicity Analysis
3.4.2. Effect of RTVH-B and FRTVH-V on In Vitro Iron Bioavailability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Li, Y.; Clark, K.A.; Tan, Z. Methods for engineering therapeutic peptides. Chin. Chem. Lett. 2018, 29, 1074–1078. [Google Scholar] [CrossRef]
- Guo, L.; Hou, H.; Li, B.; Zhang, Z.; Wang, S.; Zhao, X. Preparation, isolation and identification of iron-chelating peptides derived from Alaska pollock skin. Process Biochem. 2013, 48, 988–993. [Google Scholar] [CrossRef]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.; Moses, J.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wu, C.-H.; Yang, J.-I.; Li, Y.-H.; Kuo, J.-M. Evaluation of iron-binding activity of collagen peptides prepared from the scales of four cultivated fishes in Taiwan. J. Food Drug Anal. 2015, 23, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegría, A.; Cilla, A. Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit. Rev. Food Sci. Nutr. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods 2017, 35, 418–427. [Google Scholar] [CrossRef]
- Le Vo, T.D.; Pham, K.T.; Van Le, V.M.; Lam, H.H.; Huynh, O.N.; Vo, B.C. Evaluation of iron-binding capacity, amino acid composition, functional properties of Acetes japonicus proteolysate and identification of iron-binding peptides. Process Biochem. 2020, 91, 374–386. [Google Scholar] [CrossRef]
- Choonpicharn, S.; Jaturasitha, S.; Rakariyatham, N.; Suree, N.; Niamsup, H. Antioxidant and antihypertensive activity of gelatin hydrolysate from Nile tilapia skin. J. Food Sci. Technol. 2015, 52, 3134–3139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, L.M.; Zapata, J.E. Effects of operation conditions on antioxidant and iron chelating activity of chemical hydrolysates from red tilapia (Oreochromis sp.) scales. Adv. J. Food Sci. Technol. 2018, 15, 210–217. [Google Scholar] [CrossRef]
- Eckert, E.; Lu, L.; Unsworth, L.D.; Chen, L.; Xie, J.; Xu, R. Biophysical and in vitro absorption studies of iron chelating peptide from barley proteins. J. Funct. Foods 2016, 25, 291–301. [Google Scholar] [CrossRef]
- Zhu, L.; Glahn, R.P.; Nelson, D.; Miller, D.D. Comparing soluble ferric pyrophosphate to common iron salts and chelates as sources of bioavailable iron in a Caco-2 cell culture model. J. Agric. Food Chem. 2009, 57, 5014–5019. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Cilla, A.; Bertoldo-Pacheco, M.T.; Netto, F.M.; Alegría, A. Evaluation of in vitro iron bioavailability in free form and as whey peptide-iron complexes. J. Food Compos. Anal. 2018, 68, 95–100. [Google Scholar] [CrossRef]
- Gómez, L.J.; Zapata, J.E. Efecto del nivel de grasa y velocidad de agitación en la hidrolisis enzimática de vísceras de tilapia roja (Orechromis sp.). Inf. Tecnol. 2017, 28, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Alder-Nissen, J. Determination of the DH of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Forghani, B.; Ebrahimpour, A.; Bakar, J.; Hamid, A.A.; Hassan, Z.; Saari, N. Enzyme hydrolysates from Stichopus horrens as a new source for angiotensin-converting enzyme inhibitory peptides. Evid. Based Complement. Altern. Med. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2012; pp. 65–138. [Google Scholar]
- Icontec. Alimento para Animales. Determinacion de los Contenidos de Calcio, Cobre, Hierro, Magnesio, Manganesio, Potasio, Sodio y Zinc. Metodo Usando Espectrometria de Absorcion Atomica. In Norma Técnica Colombiana, NTC 5151; Icontec: Bogotá, Colombia, 2003. [Google Scholar]
- Zhu, K.; Wang, X.; Guo, X. Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J. Funct. Foods 2015, 12, 23–32. [Google Scholar] [CrossRef]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A. Protective effect of antioxidants contained in milk-based fruit beverages against sterol oxidation products. J. Funct. Foods 2017, 30, 81–89. [Google Scholar] [CrossRef]
- Ngo, D.H.; Qian, Z.J.; Ryu, B.; Park, J.W.; Kim, S.K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Xiao, H.; Decker, E.A.; Yongsawatdigul, J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015, 167, 7–15. [Google Scholar] [CrossRef]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H2O2-induced oxidative stress in Caco-2 cells. Food Res. Int. 2018, 103, 335–344. [Google Scholar] [CrossRef] [Green Version]
- García-nebot, M.J.; Cilla, A.; Alegría, A.; Barberá, R.; Jesús, M.; Clemente, G. Effect of caseinophosphopeptides added to fruit beverages upon ferritin synthesis in Caco-2 cells. Food Chem. 2010, 122, 92–97. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. Available online: http://www.jbc.org/content/193/1/265.short (accessed on 13 January 2020). [PubMed]
- Deng, Y.; Butré, C.I.; Wierenga, P.A. Influence of substrate concentration on the extent of protein enzymatic hydrolysis. Int. Dairy J. 2018, 86, 39–48. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Cao, Z.-F.; Zhong, H. A novel process for the separation and recovery of value-added metals from manganese–silver ores by EDTA/EDTA–2Na and thiosulfate. Hydrometallurgy 2018, 178, 256–263. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Bertoldo-Pacheco, M.T.; Paes-Leme, A.F.; Netto, F.M. Iron-binding peptides from whey protein hydrolysates: Evaluation, isolation and sequencing by LC–MS/MS. Food Res. Int. 2015, 71, 132–139. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Kapila, S.; Sharma, V. Physicochemical characterization of mineral (iron/zinc) bound caseinate and their mineral uptake in Caco-2 cells. Food Chem. 2018, 257, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, O.; Zapata, J.; Gutiérrez, G. Modelamiento de la cinética de hidrólisis enzimática de proteínas del plasma bovino. Esc. Ing. Antioq. 2012, 17, 71–84. [Google Scholar]
- Mohammad, A.W.; Kumar, A.G.; Basha, R.K. Optimization of enzymatic hydrolysis of tilapia (Oreochromis Spp.) scale gelatine. Int. Aquat. Res. 2015, 7, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; Wiley-VCH I, Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Hardt, N.A.; Van der Goot, A.J.; Boom, R.M. Influence of high solid concentrations on enzymatic wheat gluten hydrolysis and resulting functional properties. J. Cereal Sci. 2013, 57, 531–536. [Google Scholar] [CrossRef]
- Morales, J.A.; Figueroa, O.A.; Zapata, J.E. Optimización de hidrólisis enzimática de la fracción globular de sangre bovina por metodología de superficie respuesta y evaluación de sus propiedades antioxidantes. Inf. Tecnol. 2017, 28, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Priscilla, V.; Zapata, J.E. Optimization of enzymatic hydrolysis of viscera proteins of rainbow trout (Oncorhynchus mykiss). Adv. J. Food Sci. Technol. 2018, 16, 292–300. [Google Scholar] [CrossRef]
- Ng, K.; Rosenberg, A. Possible coupling of chemical to structural dynamics in subtilisin BPN′ catalyzed hydrolysis. Biophys. Chem. 1991, 39, 57–68. [Google Scholar] [CrossRef]
- Butre, C.I.; Wierenga, P.A.; Gruppen, H. Effects of ionic strength on the enzymatic hydrolysis of diluted and concentrated whey protein isolate. J. Agric. Food Chem. 2012, 60, 5644–5651. [Google Scholar] [CrossRef] [PubMed]
- Butré, C.I.; Wierenga, P.A.; Gruppen, H. Influence of water availability on the enzymatic hydrolysis of proteins. Process Biochem. 2014, 49, 1903–1912. [Google Scholar] [CrossRef]
- Chomnawang, C.; Yongsawatdigul, J. Protein recovery of tilapia frame by-products by pH-shift method. J. Aquat. Food Prod. Technol. 2013, 22, 112–120. [Google Scholar] [CrossRef]
- Benjakul, S.; Yarnpakdee, S.; Senphan, T.; Halldorsdottir, S.M.; Kristinsson, H.G. Fish protein hydrolysates: Production, bioactivities, and applications. In Antioxidants and Functional Components in Aquatic Foods; Kristinsson, H.G., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 238–283. [Google Scholar] [CrossRef]
- Anal, A.K.; Noomhorm, A.; Vongsawasdi, P. Protein hydrolysates and bioactive peptides from seafood and crustacean waste: Their extraction, bioactive properties and industrial perspectives. In Marine Proteins and Peptides: Biological Activities and Applications; Kim, S., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 709–735. [Google Scholar] [CrossRef] [Green Version]
- Roslan, J.; Yunos, K.F.M.; Abdullah, N.; Kamal, S.M.M. Characterization of Fish Protein Hydrolysate from Tilapia (Oreochromis Niloticus) by-Product. Agric. Agric. Sci. Procedia 2014, 2, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.-W.; Jung, E.-Y.; Go, G.; Kim, G.-D.; Joo, S.-T.; Yang, H.-S. Optimization of hydrolysis conditions for bovine plasma protein using response surface methodology. Food Chem. 2015, 185, 106–111. [Google Scholar] [CrossRef]
- Figueroa, O.A.; Zapata, J.E.; Sánchez, C.P. Optimización de la hidrólisis enzimática de proteínas de plasma bovino. Inf. Tecnol. 2016, 27, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Roslan, J.; Mustapal, S.M.; Yunos, K.F.; Abdullah, N. Optimization of enzymatic hydrolysis of tilapia (Oreochromis niloticus) by-product using response surface methodology. Int. Food Res. J. 2015, 22, 1117–1123. [Google Scholar]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Chen, Z.; Yu, J.; Wang, F.; Wang, J. Characterization of structural and functional properties of fish protein hydrolysates from surimi processing by-products. Food Chem. 2014, 151, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, F.; Lin, H.; Wang, X. Isolation and purification of a peptide from Bullacta exarata and its impaction of apoptosis on prostate cancer cell. Mar. Drugs. 2013, 11, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caetano-Silva, M.E.; Alves, R.C.; Lucena, G.N.; Frem, R.C.G.; Bertoldo-Pacheco, M.T.; Lima-Pallone, J.A.; Netto, F.M. Synthesis of whey peptide-iron complexes: Influence of using different iron precursor compounds. Food Res. Int. 2017, 101, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Cui, P.; Jin, Z.; Wu, H.; Wang, Y.; Lin, S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017, 230, 627–636. [Google Scholar] [CrossRef]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. In-vitro antioxidant capacity and cytoprotective/cytotoxic effects upon Caco-2 cells of red tilapia (Oreochromis spp.) viscera hydrolysates. Food Res. Int. 2019, 120, 52–61. [Google Scholar] [CrossRef]
- Budseekoad, S.; Yupanqui, C.T.; Sirinupong, N.; Alashi, A.M.; Aluko, R.E.; Youravong, W. Structural and functional characterization of calcium and iron-binding peptides from mung bean protein hydrolysate. J. Funct. Foods 2018, 49, 333–341. [Google Scholar] [CrossRef]
- Eckert, E.; Bamdad, F.; Chen, L. Metal solubility enhancing peptides derived from barley protein. Food Chem. 2014, 159, 498–506. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Iron-chelating activity of chickpea protein hydrolysate peptides. Food Chem. 2012, 134, 1585–1588. [Google Scholar] [CrossRef]
- Williams, N.J.; Gan, W.; Reibenspies, J.H.; Hancock, R.D. Possible steric control of the relative strength of chelation enhanced fluorescence for Zinc(II) compared to Cadmium(II): Metal ion complexing properties of Tris(2-quinolylmethyl)amine, a crystallographic, UV−Visible, and fluorometric study. Inorg. Chem. 2009, 48, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Gianfranceschi, G.L.; Gianfranceschi, G.; Quassinti, L.; Bramucci, M. Biochemical requirements of bioactive peptides for nutraceutical efficacy. J. Funct. Foods 2018, 47, 252–263. [Google Scholar] [CrossRef]
- Cruz-Huerta, E.; Maqueda, D.M.; de la Hoz, L.; da Silva, V.S.N.; Pacheco, M.T.B.; Amigo, L.; Recio, I. Short communication: Identification of iron-binding peptides from whey protein hydrolysates using iron (III)-immobilized metal ion affinity chromatographyand reversed phase-HPLC-tandem mass spectrometry. J. Dairy Sci. 2016, 99, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, T.J.; Eide, D.J. Transport and storage of metal ions in biology. In Biological Inorganic Chemistry: Structure and Reactivity; Stiefel, J., Ed.; University Science Books: Sausalito, CA, USA, 2007; pp. 57–77. [Google Scholar]
- Argyri, K.; Miller, D.D.; Glahn, R.P.; Zhu, L.; Kapsokefalou, M. Peptides isolated from in vitro digests of milk enhance iron uptake by caco-2 cells. J. Agric. Food Chem. 2007, 55, 10221–10225. [Google Scholar] [CrossRef] [PubMed]







| Run | S (g/L) | E/S (U/g) | pH | T (°C) | DH (%) |
|---|---|---|---|---|---|
| 1 | 12 | 0.23 | 9 | 55 | 9.73 |
| 2 | 16 | 0.15 | 10 | 60 | 9.78 |
| 3 | 8 | 0.23 | 10 | 50 | 8.23 |
| 4 | 12 | 0.23 | 9 | 55 | 11.48 |
| 5 | 12 | 0.23 | 9 | 55 | 9.48 |
| 6 | 12 | 0.23 | 9 | 65 | 9.81 |
| 7 | 12 | 0.08 | 9 | 55 | 6.34 |
| 8 | 16 | 0.31 | 8 | 50 | 8.94 |
| 9 | 12 | 0.23 | 9 | 55 | 7.69 |
| 10 | 16 | 0.15 | 8 | 50 | 8.88 |
| 11 | 8 | 0.15 | 10 | 60 | 8.95 |
| 12 | 8 | 0.31 | 10 | 60 | 18.68 |
| 13 | 8 | 0.15 | 8 | 50 | 7.21 |
| 14 | 12 | 0.23 | 9 | 45 | 8.33 |
| 15 | 16 | 0.31 | 10 | 60 | 13.06 |
| 16 | 12 | 0.38 | 9 | 55 | 10.74 |
| 17 | 4 | 0.23 | 9 | 55 | 9.41 |
| 18 | 12 | 0.23 | 9 | 55 | 8.99 |
| 19 | 20 | 0.23 | 9 | 55 | 7.63 |
| 20 | 16 | 0.31 | 10 | 50 | 11.80 |
| 21 | 12 | 0.23 | 9 | 55 | 9.61 |
| 22 | 8 | 0.31 | 8 | 60 | 11.78 |
| 23 | 16 | 0.15 | 10 | 50 | 7.90 |
| 24 | 8 | 0.31 | 8 | 50 | 11.07 |
| 25 | 16 | 0.31 | 8 | 60 | 5.28 |
| 26 | 16 | 0.15 | 8 | 60 | 6.48 |
| 27 | 12 | 0.23 | 7 | 55 | 5.89 |
| 28 | 8 | 0.31 | 10 | 50 | 16.69 |
| 29 | 8 | 0.15 | 8 | 60 | 6.47 |
| 30 | 12 | 0.23 | 11 | 55 | 14.76 |
| Source Model | F Value | p-Value |
|---|---|---|
| Model | 23.106 | <0.0001 |
| A-S | 11.896 | 0.0023 |
| B-ES | 50.195 | <0.0001 |
| C-pH | 61.519 | <0.0001 |
| D-T | 0.209 | 0.6523 |
| AB | 19.224 | 0.0002 |
| BC | 12.682 | 0.0017 |
| CD | 6.015 | 0.0226 |
| Lack of Fit | 0.982 | 0.5624 |
| R2 | 0.880 | |
| Adjusted R2 | 0.842 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. Optimization of the Red Tilapia (Oreochromis spp.) Viscera Hydrolysis for Obtaining Iron-Binding Peptides and Evaluation of In Vitro Iron Bioavailability. Foods 2020, 9, 883. https://doi.org/10.3390/foods9070883
Gómez LJ, Gómez NA, Zapata JE, López-García G, Cilla A, Alegría A. Optimization of the Red Tilapia (Oreochromis spp.) Viscera Hydrolysis for Obtaining Iron-Binding Peptides and Evaluation of In Vitro Iron Bioavailability. Foods. 2020; 9(7):883. https://doi.org/10.3390/foods9070883
Chicago/Turabian StyleGómez, Leidy J., Nathalia A. Gómez, José E. Zapata, Gabriel López-García, Antonio Cilla, and Amparo Alegría. 2020. "Optimization of the Red Tilapia (Oreochromis spp.) Viscera Hydrolysis for Obtaining Iron-Binding Peptides and Evaluation of In Vitro Iron Bioavailability" Foods 9, no. 7: 883. https://doi.org/10.3390/foods9070883
APA StyleGómez, L. J., Gómez, N. A., Zapata, J. E., López-García, G., Cilla, A., & Alegría, A. (2020). Optimization of the Red Tilapia (Oreochromis spp.) Viscera Hydrolysis for Obtaining Iron-Binding Peptides and Evaluation of In Vitro Iron Bioavailability. Foods, 9(7), 883. https://doi.org/10.3390/foods9070883