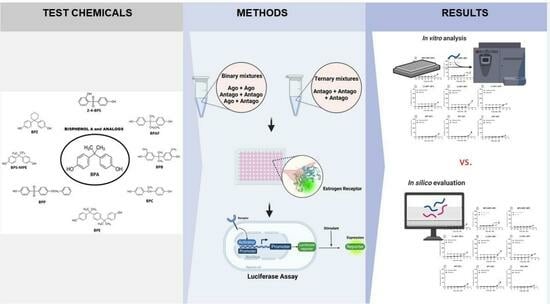
Mixture Effects of Bisphenol A and Its Structural Analogs on Estrogen Receptor Transcriptional Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Chemicals
2.2. Cell Culture
2.3. Cytotoxicity Test
2.4. Preparation of Mixtures
2.5. Stably Transfected Transactivation (STTA) Assay for Estrogen Receptor Alpha
2.6. The Prediction of ERα Transactivation of the Mixtures by the Full Logistic Model
- MAX: the maximal value of the dose–response curve in the test of individual chemicals;
- MIN: the minimal value of the dose–response curve in the test of individual chemicals;
- EC50: the half value of maximal effective concentration;
- P: hill slope;
- C: the concentration of individual chemicals in the test mixture;
- The number indicates individual chemicals in the test mixture.
2.7. The Assessment of Model Deviation Ratio (MDR)
3. Results
3.1. Cell Viability and Test Concentration
3.2. Effects of Bisphenol A and Its Analogs on ERα Transactivation: Agonist Assay
3.3. Effects of Bisphenol A and Its Analogs on ERα Transactivation: Antagnoist Assay
3.4. ERα Transactivation of Binary Mixtures
3.4.1. Mixtures of Agonist/Agonist
3.4.2. Mixtures of Antagonist/Antagonist
3.5. ERα Transactivation of Ternary Mixtures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2020. [Google Scholar] [CrossRef] [PubMed]
- Meenu Maniradhan, M.; Calivarathan, L. Bisphenol A-Induced Endocrine Dysfunction and its Associated Metabolic Disorders. Endocr. Metab. Immune Disord. -Drug Targets 2023, 23, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.E.; Cairrao, E. Effect of bisphenol A on the neurological system: A review update. Arch. Toxicol. 2023. [Google Scholar] [CrossRef]
- Guo, J.; Liu, K.; Yang, J.; Su, Y. Prenatal exposure to bisphenol A and neonatal health outcomes: A systematic review. Environ. Pollut. 2023, 335, 122295. [Google Scholar] [CrossRef]
- Fonseca, M.I.; Lorigo, M.; Cairrao, E. Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review. J. Xenobiot. 2022, 12, 181–213. [Google Scholar] [CrossRef]
- Rocca, Y.D.; Traini, E.M.; Diomede, F.; Fonticoli, L.; Trubiani, O.; Paganelli, A.; Pizzicannella, J.; Marconi, G.D. Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions. Pharmaceutics 2023, 15, 908. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef] [PubMed]
- Sabour, A.; Helou, M.E.; Roger-Leroi, V.; Bauer, C. Release and toxicity of bisphenol-A (BPA) contained in orthodontic adhesives: A systematic review. Int. Orthod. 2021, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K. Concern about the Safety of Bisphenol A Substitutes. Diabetes Metab. J. 2019, 43, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Keminer, O.; Teigeler, M.; Kohler, M.; Wenzel, A.; Arning, J.; Kaßner, F.; Windshügel, B.; Eilebrecht, E. A tiered high-throughput screening approach for evaluation of estrogen and androgen receptor modulation by environmentally relevant bisphenol A substitutes. Sci. Total Environ. 2020, 717, 134743. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Grimaldi, M.; Pons, J.; Boulahtouf, A.; Maire, A.; Vincent Cavailles, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. PNAS 2012, 109, 4930–14935. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Qiu, W.; Zhan, H.; Hu, J.; Zhang, T.; Xu, H.; Wong, M.; Xu, B.; Zheng, C. The occurrence, potential toxicity, and toxicity mechanism of bisphenol S, a substitute of bisphenol A: A critical review of recent progress. Ecotoxicol. Environ. Saf. 2019, 173, 192–202. [Google Scholar] [CrossRef]
- Téteau, O.; Vitorino Carvalho, A.; Papillier, P.; Mandon-Pépin, B.; Jouneau, L.; Jarrier-Gaillard, P.; Desmarchais, A.; Lebachelier de la Riviere, M.E.; Vignault, C.; Maillard, V.; et al. Bisphenol A and bisphenol S both disrupt ovine granulosa cell steroidogenesis but through different molecular pathways. J. Ovarian Res. 2023, 16, 30. [Google Scholar] [CrossRef]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Liang, S.; Yin, L.; Yu, S.K.; Hofmann, M.C.; Yu, X. High-Content Analysis Provides Mechanistic Insights into the Testicular Toxicity of Bisphenol A and Selected Analogues in Mouse Spermatogonial Cells. Toxicol. Sci. 2017, 155, 43–60. [Google Scholar] [CrossRef]
- Gély, C.A.; Lacroix, M.Z.; Roques, B.B.; Toutain, P.L.; Gayrard, V.; Picard-Hagen, N. Comparison of toxicokinetic properties of eleven analogues of Bisphenol A in pig after intravenous and oral administrations. Environ. Int. 2023, 171, 107722. [Google Scholar] [CrossRef] [PubMed]
- In, S.; Yoon, H.W.; Yoo, J.W.; Cho, H.; Kim, R.O.; Lee, Y.M. Acute toxicity of bisphenol A and its structural analogues and transcriptional modulation of the ecdysone-mediated pathway in the brackish water flea Diaphanosoma celebensis. Ecotoxicol. Environ. Saf. 2019, 179, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Hend, F.; Alharbi, H.F.; Algonaiman, R.; Alduwayghiri, R.; Aljutaily, T.; Algheshairy, R.M.; Almutairi, A.S.; Alhar, R.M. Exposure to Bisphenol A Substitutes, Bisphenol S and Bisphenol F, and Its Association with Developing Obesity and Diabetes Mellitus: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15918. [Google Scholar] [CrossRef]
- Mendy, A.; Salo, P.M.; Wilkerson, J.; Feinstein, L.; Ferguson, K.K.; Fessler, M.B.; Thorne, P.S.; Zeldin, D.C. Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes. Environ. Res. 2020, 183, 108944. [Google Scholar] [CrossRef]
- Ribeiro, E.; Ladeira, C.; Viegas, S. EDCs Mixtures: A Stealthy Hazard for Human Health? Toxics 2017, 5, 5. [Google Scholar] [CrossRef]
- Park, C.; Song, H.; Choi, J.; Sim, S.; Kojima, H.; Park, J.; Iida, M.; Lee, Y. The mixture effects of bisphenol derivatives on estrogen receptor and androgen receptor. Environ. Pollut. 2020, 260, 114036. [Google Scholar] [CrossRef] [PubMed]
- Skledar, D.G.; Mašič, L.P. In vitro estrogenic activity of binary and multicomponent mixtures with bisphenol A. Sci. Total Environ. 2020, 707, 135211. [Google Scholar] [CrossRef]
- Mao, W.; Song, Y.; Sui, H.; Cao, P.; Liu, Z. Analysis of individual and combined estrogenic effects of bisphenol, nonylphenol and diethylstilbestrol in immature rats with mathematical models. Environ. Health Prev. Med. 2019, 24, 32. [Google Scholar] [CrossRef]
- Baralić, K.; Buha Djordjevic, A.; Živančević, K.; Antonijević, E.; Anđelković, M.; Javorac, D.; Ćurčić, M.; Bulat, Z.; Antonijević, B.; Đukić-Ćosić, D. Toxic Effects of the Mixture of Phthalates and Bisphenol A-Subacute Oral Toxicity Study in Wistar Rats. Int J. Environ. Res. Public Health 2020, 17, 746. [Google Scholar] [CrossRef]
- Yang, J.; Liao, A.; Hu, S.; Zheng, Y.; Liang, S.; Han, S.; Lin, Y. Acute and Chronic Toxicity of Binary Mixtures of Bisphenol A and Heavy Metals. Toxics 2022, 10, 255. [Google Scholar] [CrossRef]
- OECD. OECD TG 455. Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists; OECD: Paris, France, 2021. [Google Scholar]
- Lee, H.; Park, J.; Park, K. Effects of consumer products chemicals ingredients and their mixtures on the estrogen receptor/androgen receptor transcriptional activation. Chemosphere 2022, 302, 134866. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Takeyoshi, M.; Yakabe, Y.; Sawaki, M.; Imatanaka, N.; Takatsuki, M. Comparison of reporter gene assay and immature rat uterotrophic assay of twenty-three chemicals. Toxicology 2002, 170, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ezechiáš, M.; Cajthaml, T. Novel full logistic model for estimation of the estrogenic activity of chemical mixtures. Toxicology 2016, 359–360, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Ezechiáš, M.; Cajthaml, T. Receptor partial agonism and method to express receptor partial activation concerning novel Full Logistic Model of mixture toxicology. Toxicology 2018, 393, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Michalíková, K.; Linhartová, L.; Ezechiáš, M.; Cajthaml, T. Assessment of agonistic and antagonistic properties of widely used oral care antimicrobial substances toward steroid estrogenic and androgenic receptors. Chemosphere 2018, 217, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Kudłak, B.; Simeonov, V.; Mazerska, Z.; Namieśnik, J. Binary Mixtures of Selected Bisphenols in the Environment: Their Toxicity in Relationship to Individual Constituents. Molecules 2018, 23, 3226. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wieczerzak, M.; Namieśnik, J. Bisphenols (A, S, and F) affect the basic hormonal activity determined for pharmaceuticals—Study of Saccharomyces cerevisiae. Environ. Pollut. 2019, 246, 914–920. [Google Scholar] [CrossRef]
- Li, Y.; Burns, K.A.; Arao, Y.; Luh, C.J.; Korach, K.S. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ. Health Perspect. 2012, 120, 1029–1035. [Google Scholar] [CrossRef]
- Liu, X.; Suyama, K.; Nose, T.; Shimohigashi, M.; Shimohigashi, Y. Bisphenol-C is the strongest bifunctional ERα-agonist and ERβ-antagonist due to magnified halogen bonding. PLoS ONE 2021, 16, e0246583. [Google Scholar] [CrossRef]
- Liu, X.; Matsuyama, Y.; Shimohigashi, M.; Shimohigashi, Y. Toxicol ERα-agonist and ERβ-antagonist bifunctional next-generation bisphenols with no halogens: BPAP, BPB, and BPZ. Toxicol. Lett. 2021, 345, 24–33. [Google Scholar] [CrossRef]
- Cao, L.Y.; Ren, X.M.; Li, C.H.; Zhang, J.; Qin, W.P.; Yang, Y.; Wan, B.; Guo, L.H. Bisphenol AF and Bisphenol B Exert Higher Estrogenic Effects than Bisphenol A via G Protein-Coupled Estrogen Receptor Pathway. Environ. Sci. Technol. 2017, 51, 11423–11430. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Ikhlas, S.; Ahmad, M. Occurrence, toxicity and endocrine disrupting potential of Bisphenol B and Bisphenol F: A mini-review. Toxicol. Lett. 2019, 312, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, L.; Fan, D.; Wang, Z.; Gu, W.; Shi, L.; Liu, J.; Yang, J. Bisphenol P activates hormonal genes and introduces developmental outcomes in Chironomus tentans. Ecotoxicol. Environ. Saf. 2019, 174, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, E.; Park, E.K.; Lee, S.; Kwon, J.A.; Shin, B.H.; Kang, S.; Park, E.Y.; Kim, B. Urinary Concentrations of Bisphenol Mixtures during Pregnancy and Birth Outcomes: The MAKE Study. Int. J. Environ. Res. Public. Health. 2021, 18, 10098. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Martin, O.; Ermler, S.; Baig, A.; Scholze, M. Bisphenol A and declining semen quality: A systematic review to support the derivation of a reference dose for mixture risk assessments. Int. J. Hyg. Environ. Health 2022, 241, 113942. [Google Scholar] [CrossRef]
- Zhan, W.; Tang, W.; Shen, X.; Xu, H.; Zhang, J. Exposure to bisphenol A and its analogs and polycystic ovarian syndrome in women of childbearing age: A multicenter case-control study. Chemosphere 2023, 313, 137463. [Google Scholar] [CrossRef]






| No. | Chemical Name | CAS No. | M.W. | Test Con. (M) | Vendors | Structure |
|---|---|---|---|---|---|---|
| 1 | 4,4′-Isopropylidenediphenol (BPA) | 80-05-7 | 228.29 | 10−11–10−5 | A |  |
| 2 | 4,4′-(Hexafluoroisopropylidene)diphenol (BPAF) | 1478-61-1 | 336.23 | 10−11–10−5 | A |  |
| 3 | 2,2-Bis(4-hydroxyphenyl)butane (BPB) | 77-40-7 | 242.32 | 10−11–10−5 | B |  |
| 4 | 2,2-Bis(4-hydroxy-3-methylphenyl)propane (BPC) | 79-97-0 | 256.35 | 10−11–10−5 | B |  |
| 5 | 4,4′-Ethylidenediphenol (BPE) | 2081-08-05 | 214.26 | 10−11–10−5 | B |  |
| 6 | α,α′-Bis(4-hydroxyphenyl)-1,4-diisopropylbenzene (BPP) | 2167-51-3 | 346.46 | 10−11–10−6 | B |  |
| 7 | 4-Benzyloxyphenyl 4-Hydroxyphenyl Sulfone (BPS-MPE) | 63134-33-8 | 340.39 | 10−11–10−6 | B |  |
| 8 | 4,4′-Cyclohexylidenebisphenol (BPZ) | 843-55-0 | 268.35 | 10−11–10−5 | A |  |
| 9 | 2,4′-Dihydroxydiphenyl Sulfone (2,4-BPS) | 5397-34-2 | 250.27 | 10−11–10−6 | B |  |
| Chemicals | EC50 | IC50 | MIN | MAX | Hill Slope | |||
|---|---|---|---|---|---|---|---|---|
| (M) | (Log M) | (M) | (Log M) | |||||
| Agonist | BPA | 2.31 × 10−7 | −6.64 | - | - | 2.66 | 95.14 | 0.66 |
| BPAF | 2.40 × 10−7 | −6.62 | - | - | 5.00 | 93.34 | 1.11 | |
| BPB | 2.72 × 10−4 | −3.57 | - | - | −6.41 | 424.1 | 0.32 | |
| BPC | 2.91 × 10−7 | −6.54 | - | - | −3.95 | 180.3 | 0.45 | |
| BPE | 1.04 × 10−7 | −6.98 | - | - | 2.12 | 120.6 | 7.36 | |
| BPZ | 3.26 × 10−7 | −6.49 | - | - | 4.04 | 108.5 | 1.52 | |
| Antagonist | BPP | - | - | 1.62 × 10−7 | −6.79 | −5.64 | 92.12 | −0.56 |
| BPS-MPE | - | - | 1.63 × 10−7 | −6.79 | −4.52 | 96.26 | −0.51 | |
| 2,4-BPS | - | - | 1.50 × 10−8 | −7.83 | 19.75 | 93.86 | −0.40 | |
| Chemicals | Ind. Conc. (M) | Total Conc. | Ratio | |||
|---|---|---|---|---|---|---|
| (M) | (Log M) | |||||
| Binary | Ago + Ago | BPA + BPAF | 2.3 × 10−5 + 2.4 × 10−5 | 4.7 × 10−5 | −4.33 | 1.0:1.0 |
| BPA + BPB (1) | 1.2 × 10−8 +1.4 × 10−5 | 1.4 × 10−5 | −4.87 | 1.0:1174.6 | ||
| BPA + BPC | 2.3 × 10−5 + 2.9 × 10−5 | 5.2 × 10−5 | −4.28 | 1.0:1.3.0 | ||
| BPA + BPE | 2.3 × 10−5 + 1.1 × 10−5 | 3.4 × 10−5 | −4.47 | 1.0:2.2 | ||
| BPA + BPZ | 2.3 × 10−5 + 3.3 × 10−5 | 5.6 × 10−5 | −4.25 | 1.0:1.4 | ||
| Anta + Anta | BPS-MPE + BPP | 1.6 × 10−5 + 1.6 × 10−5 | 3.3 × 10−5 | −4.49 | 1.0:1.0 | |
| 2,4-BPS + BPP | 1.5 × 10−6 + 1.6 × 10−5 | 1.8 × 10−5 | −4.75 | 1.0:10.8 | ||
| 2,4-BPS + BPS-MPE | 1.5 × 10−6 + 1.6 × 10−5 | 1.8 × 10−5 | −4.75 | 1.0:10.9 | ||
| Ternary | Ago + Ago + Ago | BPA + BPAF + BPZ | 2.3 × 10−5 + 2.4 × 10−5 + 3.3 × 10−5 | 8.0 × 10−5 | −4.10 | 1.0:1.0:1.4 |
| BPAF + BPZ + BPB (2) | 6.0 × 10−8 + 8.1 × 10−8 + 6.8 × 10−5 | 6.8 × 10−5 | −4.17 | 1.0:1.4:1131.6 | ||
| BPE + BPC + BPB (2) | 2.6 × 10−8 + 7.3 × 10−8 + 6.8 × 10−5 | 6.8 × 10−5 | −4.17 | 1.0:2.8:2611.0 | ||
| BPE + BPA + BPC | 1.0 × 10−5 + 2.3 × 10−5 + 2.9 × 10−5 | 6.3 × 10−5 | −4.20 | 1.0:2.2:2.8 | ||
| BPC + BPZ + BPB (2) | 7.3 × 10−8 + 8.1 × 10−8 + 6.8 × 10−5 | 6.8 × 10−5 | −4.17 | 1.0:1.1:935.6 | ||
| Anta + Anta + Anta | 2,4-BPS + BPP + BPS-MPE | 1.5 × 10−6 + 1.6 × 10−5 + 1.6 × 10−5 | 3.4 × 10−5 | −4.47 | 1.0:10.8:10.9 | |
| Chemicals | RPCMax (%) | PC10 | PC50 | IC30 | IC50 | |
|---|---|---|---|---|---|---|
| (Log M) | (Log M) | (Log M) | (Log M) | |||
| Agonist | BPA | 88.35 | −8.36 | −6.63 | - | - |
| BPAF | 91.34 | −8.35 | −6.57 | - | - | |
| BPB | 104.53 | −8.16 | −6.00 | - | - | |
| BPC | 148.20 | −9.24 | −7.33 | - | - | |
| BPE | 138.06 | −8.42 | −7.07 | - | - | |
| BPZ | 107.87 | −7.87 | −6.58 | - | - | |
| Antagonist | BPP | - | - | - | −7.39 | −6.68 |
| BPS-MPE | - | - | - | −7.57 | −6.74 | |
| 2,4-BPS | - | - | - | −8.84 | −8.00 | |
| Chemicals | RPCMax (%) | PC10 (Log M) | PC50 (Log M) | IC30 (Log M) | IC50 (Log M) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meas | Pred | Meas | Pred | Meas | Pred | Meas | Pred | Meas | Pred | ||
| Ago + Ago | BPA + BPAF | 96.7 | 92.8 | −9.43 | −8.16 | −6.74 | −6.63 | - | - | - | - |
| BPA + BPB | 134.6 | 146.0 | −6.79 | <−7.56 | −5.78 | −5.54 | - | - | - | - | |
| BPA + BPC | 121.6 | 128.7 | −9.12 | −8.84 | −7.22 | −7.06 | - | - | - | - | |
| BPA + BPE | 96.8 | 106.5 | −7.97 | −8.00 | −6.44 | −6.86 | - | - | - | - | |
| BPA + BPZ | 100.8 | 100.4 | −9.72 | −8.00 | −6.44 | −6.65 | - | - | - | - | |
| Anta + Anta | BPS-MPE + BPP | 101.2 | 90.7 | - | - | - | - | −7.35 | −7.77 | −6.26 | −6.98 |
| 2,4-BPS + BPP | 98.8 | 88.7 | - | - | - | - | −8.01 | −8.00 | −7.16 | −7.06 | |
| BPS-MPE + 2,4-BPS | 99.0 | 90.2 | - | - | - | - | −8.10 | −7.94 | −7.26 | −7.00 | |
| Chemicals | RPCMax (%) | PC10 (Log M) | PC50 (Log M) | IC30 (Log M) | IC50 (Log M) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Meas | Pred | Meas | Pred | Meas | Pred | Meas | Pred | Meas | Pred | |
| BPA + BPAF + BPZ | 106.1 | 98.2 | −8.50 | −7.95 | −6.33 | −6.61 | - | - | - | - |
| BPAF + BPZ + BPB | 104.1 | 95.4 | −8.00 | −7.73 | −5.60 | −5.17 | - | - | - | - |
| BPB + BPC + BPE | 97.9 | 89.7 | −8.00 | −7.23 | −5.03 | −5.15 | - | - | - | - |
| BPC + BPE + BPA | 129.8 | 127.0 | −8.53 | −8.61 | −6.40 | −6.94 | - | - | - | - |
| BPZ + BPB + BPC | 105.8 | 108.7 | −8.26 | −7.51 | −5.48 | −5.40 | - | - | - | - |
| BPS-MPE + 2,4-BPS + BPP | 97.6 | 89.1 | - | - | - | - | −7.65 | −7.91 | −6.71 | −7.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Park, J.; Park, K. Mixture Effects of Bisphenol A and Its Structural Analogs on Estrogen Receptor Transcriptional Activation. Toxics 2023, 11, 986. https://doi.org/10.3390/toxics11120986
Lee H, Park J, Park K. Mixture Effects of Bisphenol A and Its Structural Analogs on Estrogen Receptor Transcriptional Activation. Toxics. 2023; 11(12):986. https://doi.org/10.3390/toxics11120986
Chicago/Turabian StyleLee, Handule, Juyoung Park, and Kwangsik Park. 2023. "Mixture Effects of Bisphenol A and Its Structural Analogs on Estrogen Receptor Transcriptional Activation" Toxics 11, no. 12: 986. https://doi.org/10.3390/toxics11120986
APA StyleLee, H., Park, J., & Park, K. (2023). Mixture Effects of Bisphenol A and Its Structural Analogs on Estrogen Receptor Transcriptional Activation. Toxics, 11(12), 986. https://doi.org/10.3390/toxics11120986