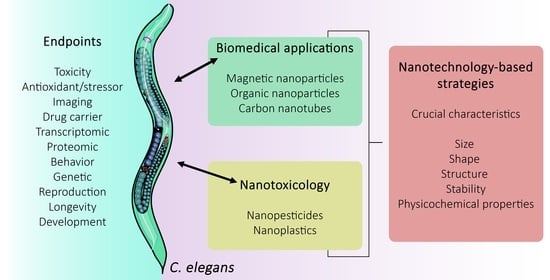
Caenorhabditis elegans as a Prediction Platform for Nanotechnology-Based Strategies: Insights on Analytical Challenges
Abstract
:1. Introduction
2. Nanomaterials
2.1. An Overview
2.2. Nanotoxicity
2.3. Assessment and Regulation
3. Caenorhabditis elegans
4. Caenorhabditis elegans and Magnetic Nanoparticles
5. Caenorhabditis elegans and Organic Nanoparticles
5.1. Liposomes
5.2. Nanoemulsion
5.3. Dendrimers
6. Caenorhabditis elegans and Carbon Nanosystems
6.1. Multiwalled Carbon Nanotubes
6.2. Single-Walled Carbon Nanotubes
6.3. Fullerenes and Graphene Quantum Dots
6.4. Graphene Oxide
| Multiwalled Carbon Nanotubes (MWCNT) | ||||
|---|---|---|---|---|
| Concentrations/Time | Exposure Conditions | Endpoint | Effects | Reference |
| 100 mg L−1 for 24 h 50 mg L−1 for 72 h | K-medium E. coli OP50 Young adults (day-3) | Survival | No changes | [127] |
| Reproduction | Decrease (only pristine coated) | |||
| 100–500 mg L−1 for 3–15 days | NMG medium surface (5.203 mg L−1 FUDR, E. coli OP50). L4 until death | Survival | No changes | [129] |
| Movement | Decreased (100 mg L−1, 500 mg L−1) | |||
| 0.1 μg L−1 | NMG medium surface E. coli OP50 L1-Day-1 Adult | Gene regulation | 149 genes up-regulated and 193 genes down-regulated | [125] |
| 1010 particles per mL for 72 h | NMG medium surface E. coli OP50 L1-Day-1 Adult | Transcriptome | Upregulated daf-2 (3.18) daf-16 (1.01) bet-1 (2.48) rpl-33 (1.01) rps-14 (1.01) rps-20 (1.01) par-1 (1.01) lin-45 (1.01) lag-1 (1.01) dnj-10 (2.12) Gene suppression smk-1 (−2.13) rpl-31 (−6.50) ubq-2 (−2.08) daf-12 (−5.15) spp-1 (−2.44) hsp-60 (−2.17) | [112] |
| Survival | No changes | |||
| Body length | No changes | |||
| Single-walled carbon nanotubes (SWCNTs) SWCNTs−COOH | ||||
| Concentrations/Time | Exposure conditions | Endpoint | Effects | Reference |
| 0.001 to 1000 μg L−1 SWCNTs− COOH for 24 h (acute exposure) | NMG medium surface L3/young L4 | Lethality | No changes | [120] |
| Lifespan | Decrease | |||
| Growth | Decrease | |||
| Reproduction | Decrease | |||
| Locomotion | Decrease | |||
| ROS | Increase | |||
| Antioxidant system | Increase the expression of sod-3, ctl-2, and cyp-35A2 | |||
| 0.1–300 mg L−1 SWCNT and ssDNA-SWCNT for 4–24 h. | NMG medium surface or vials (NaCl 0.9%). Day-1 Adult | Reproduction | No changes | [134] |
| Survival | No changes | |||
| Imaging acquisition | Fluorescent bio-imaging | |||
| 50, 100, and 250 mg mL−1 Cys–SWNTs for 3 h | NMG medium surface Suspensions of Cys–SWNTs in M9 buffer L4 | Survival | No changes | [133] |
| Lifespan | No changes | |||
| Brood-size | No changes | |||
| 100, 250, and 500 mg mL−1 a-SWCNTs for 48 h | NMG medium surface L1-Day-1 Adult | Survival | No changes | [132] |
| Lifespan | Decrease | |||
| Reproduction | Decrease | |||
| Expression of the BOW phenotype | Activated | |||
| Fullerenes and Graphene quantum dots | ||||
| Concentrations/Time | Exposure conditions | Endpoint | Effects | Reference |
| 100 µg mL−1 hydroxylated fullerene (fullerol) | NMG medium surface E. coli OP50 L4- Day-5 Adult | Lifespan | Decrease | [136] |
| Eggs laid | Decrease | |||
| Body size | Decrease | |||
| Apoptotic process | Increase | |||
| 0.01 and 100 µM polyhydroxylated fullerene (fullerenol) for 24 h | NMG medium surface E. coli OP50 L1 | Autofluorescence | Decrease | [137] |
| 200 μg mL−1 N-GQD for 24 h | K medium L4-Day-1 Adult | Neurotoxicity | Increase | [139] |
| Graphene Oxide | ||||
| Concentrations/Time | Exposure conditions | Endpoint | Effects | Reference |
| 100 mg L−1 >1 mg L−1 for 24 h | NMG medium surface E. coli OP50 L4 | Body length | Decrease | [85] |
| Lifespan | Decrease | |||
| Brood size | Increase | |||
| 1–100 mg L−1 for 48 h | NGM medium surface E. coli OP50 L1- young adults | Survival | No changes | [141] |
| Expression pattern of genes encoding p38 MAPK signaling | Increase pmk-1, sek-1, and nsy-1 Decrease PMK-1::GFP expression in sek-1(ag1) or nsy-1(ag3) mutants | |||
| >100 μg L−1 Thiolated graphene oxide | K-medium E. coli OP50 L1- Day-1 Adult | Reproduction | Decrease | [144] |
| Locomotion | Decrease | |||
| ROS production | Increase | |||
| Intestinal permeability | Increase | |||
| 10 mg L−1 for 48 h | NMG medium surface K-medium L1- L4 larval stage | Uptake | at 2 h | [143] |
| Bioaccumulation | at 48 h (reproductive system) | |||
| Reproduction | Impairment, suppression of spermatogenesis | |||
| Oxidative stress | Increase | |||
| Fat accumulation | Increase | |||
| 10–100 mg L−1 for 48 h | NMG medium surface E. coli OP50 L1–L4 larval stage | Autophagy | Increase | [142] |
7. Caenorhabditis elegans and Nanopesticides
8. Caenorhabditis elegans and Nanoplastics
8.1. Nanoplastic Definition
8.2. Nanoplastics and the Risks to Human Health
8.3. Lack of Studies Using Environmental Nanomaterial
8.4. Studying Nanoplastic Toxicity Using Caenorhabditis elegans
Nanopolystyrene and Caenorhabditis elegans
9. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-MA | 3-methyladenine |
| C. elegans | Caenorhabditis elegans |
| CAT | Catalase |
| COVID-19 | Coronavirus disease |
| E. coli | Escherichia coli |
| EMA | European Medicines Agency |
| FDA | Food and Drugs Agency |
| fMWNTs | Acid-functionalized MWCNTs |
| GRK2 | G protein-coupled receptor kinase |
| HDFM | Hyperspectral dark field microscopy |
| HIV/AIDS | Human immunodeficiency virus/acquired immunodeficiency syndrome |
| MAPK | Mitogen-activated protein kinase |
| MNPs | Magnetic nanoparticles |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger ribonucleic acid |
| mt UPR | Mitochondrial unfolded protein response |
| MWCNTs | Multiwalled carbon nanotubes |
| N-GQD | Nitrogen-doped graphene quantum dots |
| NAC | N-Acetyl-L-cysteine |
| NIR | Near-infrared |
| PAMAM | Poly(amidoamine) dendrimers |
| PEG | Polyethylene glycol |
| PLA | Polymeric polylactic acid |
| PRDx | Peroxiredoxin-2 |
| ROS | Reactive oxygen species |
| SAR | Specific absorption rate |
| SOD | Superoxide dismutase |
| SWCNTs | Single-walled carbon nanotubes |
| TEM | Transmission electron microscopy |
| μ-SRXRF | Synchrotron radiation X-ray fluorescence |
References
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Dalvi, S.V.; Siril, P.F. Nanoparticle-Based Drugs and Formulations: Current Status and Emerging Applications. ACS Appl. Nano Mater. 2020, 3, 4944–4961. [Google Scholar] [CrossRef]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic Nanoparticle Hyperthermia In Cancer Treatment. Nano Life 2010, 01, 17–32. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Purayil, S.K.; Ponnaiah, P. A Review on Synthesis, Characterization and Potential Biological Applications of Superparamagnetic Iron Oxide Nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral Formulation Development and Biopharmaceutical Evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Müller, R. Junghanns Nanocrystal Technology, Drug Delivery and Clinical Applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, R. Nanotechnology-Based Drug Delivery Systems. NanoBiotechnology 2009, 5, 17–33. [Google Scholar] [CrossRef]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization Strategies for Poorly Water-Soluble Drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef]
- Idée, J.-M.; Louguet, S.; Ballet, S.; Corot, C. Theranostics and Contrast-Agents for Medical Imaging: A Pharmaceutical Company Viewpoint. Quant. Imaging. Med. Surg. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Pene, F.; Courtine, E.; Cariou, A.; Mira, J.-P. Toward Theragnostics: Crit. Care Med. 2009, 37, S50–S58. [Google Scholar] [CrossRef]
- Pudlarz, A.; Szemraj, J. Nanoparticles as Carriers of Proteins, Peptides and Other Therapeutic Molecules. Open Life Sci. 2018, 13, 285–298. [Google Scholar] [CrossRef]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, Distribution, Metabolism, and Excretion of Nanocarriers in Vivo and Their Influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.-Z.; Chen, W.-Y.; Wu, C.-Y.; Chen, Y.-C. Potent Antibacterial Nanoparticles for Pathogenic Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 2046–2054. [Google Scholar] [CrossRef]
- Neal, A.L. What Can Be Inferred from Bacterium–Nanoparticle Interactions about the Potential Consequences of Environmental Exposure to Nanoparticles? Ecotoxicology 2008, 17, 362–371. [Google Scholar] [CrossRef]
- Curley, S.M.; Putnam, D. Biological Nanoparticles in Vaccine Development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef]
- Saboktakin, M. The Biological and Biomedical Nanoparticles—Synthesis and Applications. Adv. Mater. Sci. 2017, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Stanley, S. Biological Nanoparticles and Their Influence on Organisms. Curr. Opin. Biotechnol. 2014, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A. Protein-Nanoparticle Interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle Uptake by the Rat Gastrointestinal Mucosa: Quantitation and Particle Size Dependency. J. Pharm. Pharmacol. 2011, 42, 821–826. [Google Scholar] [CrossRef]
- Lee, J.; Park, E.Y.; Lee, J. Non-Toxic Nanoparticles from Phytochemicals: Preparation and Biomedical Application. Bioprocess Biosyst. Eng. 2014, 37, 983–989. [Google Scholar] [CrossRef]
- Pan, X.; Redding, J.E.; Wiley, P.A.; Wen, L.; McConnell, J.S.; Zhang, B. Mutagenicity Evaluation of Metal Oxide Nanoparticles by the Bacterial Reverse Mutation Assay. Chemosphere 2010, 79, 113–116. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Q.; Li, Y.; Wang, D. Translocation, Transfer, and in Vivo Safety Evaluation of Engineered Nanomaterials in the Non-Mammalian Alternative Toxicity Assay Model of Nematode Caenorhabditis elegans. RSC Adv. 2013, 3, 5741. [Google Scholar] [CrossRef]
- Wu, Q.; Nouara, A.; Li, Y.; Zhang, M.; Wang, W.; Tang, M.; Ye, B.; Ding, J.; Wang, D. Comparison of Toxicities from Three Metal Oxide Nanoparticles at Environmental Relevant Concentrations in Nematode Caenorhabditis elegans. Chemosphere 2013, 90, 1123–1131. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, B.; Wang, D. Effects of Metal Exposure on Associative Learning Behavior in Nematode Caenorhabditis elegans. Arch. Environ. Contam. Toxicol. 2010, 59, 129–136. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [Green Version]
- Werlin, R.; Priester, J.H.; Mielke, R.E.; Krämer, S.; Jackson, S.; Stoimenov, P.K.; Stucky, G.D.; Cherr, G.N.; Orias, E.; Holden, P.A. Biomagnification of Cadmium Selenide Quantum Dots in a Simple Experimental Microbial Food Chain. Nat. Nanotechnol. 2011, 6, 65–71. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An Overview on Manufactured Nanoparticles in Plants: Uptake, Translocation, Accumulation and Phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Cui, X.; Yin, J.; Lin, Y.; Li, N.; Wang, M.; Shen, D. Towards a Definition of Harmless Nanoparticles from an Environmental and Safety Perspective. J. Chem. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Li, W.; Little, N.; Park, J.; Foster, C.A.; Chen, J.; Lu, J. Tumor-Associated Fibroblast-Targeting Nanoparticles for Enhancing Solid Tumor Therapy: Progress and Challenges. Mol. Pharm. 2021, 18, 2889–2905. [Google Scholar] [CrossRef]
- Sulston, J.E.; Horvitz, H.R. Post-Embryonic Cell Lineages of the Nematode, Caenorhabditis elegans. Dev. Biol. 1977, 56, 110–156. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Zhao, Y.; Nurdebek, B.; Bu, Y.; Wang, D. Long-Term Exposure to Polystyrene Nanoparticles Causes Transgenerational Toxicity by Affecting the Function and Expression of MEV-1 and DAF-2 Signals in Caenorhabditis elegans. NanoImpact 2022, 26, 100403. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-C.; Yen, P.-L.; Chaikritsadakarn, A.; Huang, C.-W.; Chang, C.-H.; Liao, V.H.-C. Parental CuO Nanoparticles Exposure Results in Transgenerational Toxicity in Caenorhabditis elegans Associated with Possible Epigenetic Regulation. Ecotoxicol. Environ. Saf. 2020, 203, 111001. [Google Scholar] [CrossRef] [PubMed]
- Agarrayua, D.A.; Funguetto-Ribeiro, A.C.; Trevisan, P.; Haas, S.E.; Ávila, D.S. Safety Assessment of Different Unloaded Polymeric Nanocapsules in Caenorhabditis elegans. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109477. [Google Scholar] [CrossRef]
- Kim, W.; Underwood, R.S.; Greenwald, I.; Shaye, D.D. OrthoList 2: A New Comparative Genomic Analysis of Human and Caenorhabditis elegans Genes. Genetics 2018, 210, 445–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalfie, M.; Tu, Y.; Euskirchen, G.; Ward, W.W.; Prasher, D.C. Green Fluorescent Protein as a Marker for Gene Expression. Science 1994, 263, 802–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulme, S.E.; Whitesides, G.M. Chemistry and the Worm: Caenorhabditis elegans as a Platform for Integrating Chemical and Biological Research. Angew. Chem. Int. Ed. 2011, 50, 4774–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, L.R.; Fiedler, T.J.; Harris, T.W.; Carvalho, F.; Antoshechkin, I.; Han, M.; Sternberg, P.W.; Stein, L.D.; Chalfie, M. WormBook: The Online Review of Caenorhabditis elegans Biology. Nucleic. Acids. Res. 2007, 35, D472–D475. [Google Scholar] [CrossRef]
- Schulenburg, H.; Félix, M.-A. The Natural Biotic Environment of Caenorhabditis elegans. Genetics 2017, 206, 55–86. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Moragas, L.; Roig, A.; Laromaine, A.C. C. elegans as a Tool for in Vivo Nanoparticle Assessment. Adv. Colloid. Interface Sci. 2015, 219, 10–26. [Google Scholar] [CrossRef]
- Leung, M.C.K.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An Emerging Model in Biomedical and Environmental Toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef]
- Queirós, L.; Pereira, J.L.; Gonçalves, F.J.M.; Pacheco, M.; Aschner, M.; Pereira, P. Caenorhabditis elegans as a Tool for Environmental Risk Assessment: Emerging and Promising Applications for a “Nobelized Worm. ” Crit. Rev. Toxicol. 2019, 49, 411–429. [Google Scholar] [CrossRef]
- Moros, M.; Gonzalez-Moragas, L.; Tino, A.; Laromaine, A.; Tortiglione, C. Invertebrate Models for Hyperthermia: What We Learned From Caenorhabditis elegans and Hydra Vulgaris. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 229–264. ISBN 978-0-12-813928-8. [Google Scholar]
- Mohammadi Ziarani, G.; Malmir, M.; Lashgari, N.; Badiei, A. The Role of Hollow Magnetic Nanoparticles in Drug Delivery. RSC Adv. 2019, 9, 25094–25106. [Google Scholar] [CrossRef] [Green Version]
- Wahajuddin, N.; Arora, S. Superparamagnetic Iron Oxide Nanoparticles: Magnetic Nanoplatforms as Drug Carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-P.; Yang, P.; Lu, Y.-J.; Ma, Y.-H.; Tu, S.-J. Targeted Delivery of Tissue Plasminogen Activator by Binding to Silica-Coated Magnetic Nanoparticle. Int. J. Nanomed. 2012, 7, 5137–5149. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Current Investigations into Magnetic Nanoparticles for Biomedical Applications. J. Biomed. Mater. Res. A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent Advances in Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for in Vitro and in Vivo Cancer Nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic Hyperthermia Efficiency in the Cellular Environment for Different Nanoparticle Designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef]
- Soetaert, F.; Kandala, S.K.; Bakuzis, A.; Ivkov, R. Experimental Estimation and Analysis of Variance of the Measured Loss Power of Magnetic Nanoparticles. Sci. Rep. 2017, 7, 6661. [Google Scholar] [CrossRef] [Green Version]
- Conde-Leboran, I.; Baldomir, D.; Martinez-Boubeta, C.; Chubykalo-Fesenko, O.; del Puerto Morales, M.; Salas, G.; Cabrera, D.; Camarero, J.; Teran, F.J.; Serantes, D. A Single Picture Explains Diversity of Hyperthermia Response of Magnetic Nanoparticles. J. Phys. Chem. C 2015, 119, 15698–15706. [Google Scholar] [CrossRef]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, e629054. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.M.; Meenach, S.A.; Anderson, K.W.; Hilt, J.Z. Synthesis and Characterization of CREKA-Conjugated Iron Oxide Nanoparticles for Hyperthermia Applications. Acta Biomater. 2014, 10, 2622–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, S.K. Nanoparticles in Modern Medicine: State of the Art and Future Challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharm. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.Y.; Howarth, S.P.S.; Miller, S.R.; Graves, M.J.; Patterson, A.J.; U-King-Im, J.-M.; Li, Z.Y.; Walsh, S.R.; Brown, A.P.; Kirkpatrick, P.J.; et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. J. Am. Coll. Cardiol. 2009, 53, 2039–2050. [Google Scholar] [CrossRef]
- Gubert, G.; Gubert, P.; Sandes, J.M.; Bornhorst, J.; Alves, L.C.; Quines, C.B.; Mosca, D.H. The Nanotoxicity Assessment of Cube-like Iron Nitride Magnetic Nanoparticles at the Organismal Level of Nematode Caenorhabditis elegans. Nanotoxicology 2022, 16, 472–483. [Google Scholar] [CrossRef]
- Gubert, G.; Varalda, J.; Mosca, D.H. Effect of Wavelength and Fluence in Laser-Induced Iron Nitride Nanostructures. J. Alloys Compd. 2021, 856, 157392. [Google Scholar] [CrossRef]
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C 2019, 123, 16495–16507. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological Applications of Magnetic Nanoparticles. Chem. Soc. Rev. 2012, 41, 4306. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.-M.; Carenza, E.; Laromaine, A.; Roig, A. Protective Effects of Bovine Serum Albumin on Superparamagnetic Iron Oxide Nanoparticles Evaluated in the Nematode Caenorhabditis elegans. ACS Biomater. Sci. Eng. 2015, 1, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Minullina, R.T.; Osin, Y.N.; Ishmuchametova, D.G.; Fakhrullin, R.F. Interfacing Multicellular Organisms with Polyelectrolyte Shells and Nanoparticles: A Caenorhabtidis Elegans Study. Langmuir 2011, 27, 7708–7713. [Google Scholar] [CrossRef]
- Kumari, K.; Capstick, M.; Cassara, A.M.; Herrala, M.; Koivisto, H.; Naarala, J.; Tanila, H.; Viluksela, M.; Juutilainen, J. Effects of Intermediate Frequency Magnetic Fields on Male Fertility Indicators in Mice. Environ. Res. 2017, 157, 64–70. [Google Scholar] [CrossRef]
- Bae, J.-E.; Bang, S.; Min, S.; Lee, S.-H.; Kwon, S.-H.; Lee, Y.; Lee, Y.-H.; Chung, J.; Chae, K.-S. Positive Geotactic Behaviors Induced by Geomagnetic Field in Drosophila. Mol. Brain 2016, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Cheung, B.H.H.; Cohen, M.; Rogers, C.; Albayram, O.; de Bono, M. Experience-Dependent Modulation of C. elegans Behavior by Ambient Oxygen. Curr. Biol. 2005, 15, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Gao, X.; Zhu, J.; Zhang, T.; Xue, Y.; Tang, M. Reproductive Toxicity Induced by Nickel Nanoparticles in Caenorhabditis elegans. Environ. Toxicol. 2017, 32, 1530–1538. [Google Scholar] [CrossRef]
- Meng, F.; Ma, G.; Qiu, J.; Fu, Z.; Yan, J.; Wang, L. Facile Synthesis of Cu N-Lauroyl Sarcosinate Nanozymes with Laccase-Mimicking Activity and Identification of Toxicity Effects for C. elegans. RSC Adv. 2022, 12, 32898–32902. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Li, Y.; Zhao, Y.; Ge, L.; Wang, H.; Wang, D. Crucial Role of the Biological Barrier at the Primary Targeted Organs in Controlling the Translocation and Toxicity of Multi-Walled Carbon Nanotubes in the Nematode Caenorhabditis elegans. Nanoscale 2013, 5, 11166. [Google Scholar] [CrossRef]
- Arnold, M.C.; Badireddy, A.R.; Wiesner, M.R.; di Giulio, R.T.; Meyer, J.N. Cerium Oxide Nanoparticles Are More Toxic than Equimolar Bulk Cerium Oxide in Caenorhabditis elegans. Arch. Environ. Contam. Toxicol. 2013, 65, 224–233. [Google Scholar] [CrossRef]
- Mutwakil, M.H.A.Z.; Reader, J.P.; Holdich, D.M.; Smithurst, P.R.; Candido, E.P.M.; Jones, D.; Stringham, E.G.; de Pomerai, D.I. Use of Stress-Inducible Transgenic Nematodes as Biomarkers of Heavy Metal Pollution in Water Samples from an English River System. Arch. Environ. Contam. Toxicol. 1997, 32, 146–153. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.H.; Cha, Y.J.; Hong, S.J.; Chung, S.K.; Park, T.H.; Choi, S.S.C. C. elegans-on-a-Chip for in Situ and in Vivo Ag Nanoparticles’ Uptake and Toxicity Assay. Sci. Rep. 2017, 7, 40225. [Google Scholar] [CrossRef]
- Marimon-Bolívar, W.; Tejeda-Benítez, L.P.; Núñez-Avilés, C.A.; de Léon-Pérez, D. Evaluation of the In Vivo Toxicity of Green Magnetic Nanoparticles Using Caenorhabditis elegans as a Biological Model. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100253. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.-M.; Benseny-Cases, N.; Stürzenbaum, S.; Roig, A.; Laromaine, A. Toxicogenomics of Iron Oxide Nanoparticles in the Nematode C. elegans. Nanotoxicology 2017, 11, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.; Roh, J.; Eom, H.; Choi, J.-Y.; Hyun, J.; Choi, J. Oxidative Stress-Related PMK-1 P38 MAPK Activation as a Mechanism for Toxicity of Silver Nanoparticles to Reproduction in the Nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2012, 31, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yin, L.; Li, X.; Tang, M.; Zhang, T.; Wang, D. Contributions of Altered Permeability of Intestinal Barrier and Defecation Behavior to Toxicity Formation from Graphene Oxide in Nematode Caenorhabditis elegans. Nanoscale 2013, 5, 9934. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Delikanli, S.; Zeng, H.; Ferkey, D.M.; Pralle, A. Remote Control of Ion Channels and Neurons through Magnetic-Field Heating of Nanoparticles. Nat. Nanotechnol. 2010, 5, 602–606. [Google Scholar] [CrossRef]
- Wang, L.; Du, H.; Guo, X.; Wang, X.; Wang, M.; Wang, Y.; Wang, M.; Chen, S.; Wu, L.; Xu, A. Developmental Abnormality Induced by Strong Static Magnetic Field in Caenorhabditis elegans. Bioelectromagnetics 2015, 36, 178–189. [Google Scholar] [CrossRef]
- Ahn, J.-M.; Eom, H.-J.; Yang, X.; Meyer, J.N.; Choi, J. Comparative Toxicity of Silver Nanoparticles on Oxidative Stress and DNA Damage in the Nematode, Caenorhabditis elegans. Chemosphere 2014, 108, 343–352. [Google Scholar] [CrossRef]
- Yu, S.-M.; Gonzalez-Moragas, L.; Milla, M.; Kolovou, A.; Santarella-Mellwig, R.; Schwab, Y.; Laromaine, A.; Roig, A. Bio-Identity and Fate of Albumin-Coated SPIONs Evaluated in Cells and by the C. elegans Model. Acta Biomater. 2016, 43, 348–357. [Google Scholar] [CrossRef]
- Holmannova, D.; Borsky, P.; Svadlakova, T.; Borska, L.; Fiala, Z. Reproductive and Developmental Nanotoxicity of Carbon Nanoparticles. Nanomaterials 2022, 12, 1716. [Google Scholar] [CrossRef]
- Guo, B.; Middha, E.; Liu, B. Solvent Magic for Organic Particles. ACS Nano 2019, 13, 2675–2680. [Google Scholar] [CrossRef]
- Kumar, R.; Lal, S. Synthesis of Organic Nanoparticles and Their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 3, e1000150. [Google Scholar] [CrossRef]
- Wu, T.; Xu, H.; Liang, X.; Tang, M. Caenorhabditis elegans as a Complete Model Organism for Biosafety Assessments of Nanoparticles. Chemosphere 2019, 221, 708–726. [Google Scholar] [CrossRef]
- Mazur, F.; Bally, M.; Städler, B.; Chandrawati, R. Liposomes and Lipid Bilayers in Biosensors. Adv. Colloid. Interface Sci. 2017, 249, 88–99. [Google Scholar] [CrossRef]
- Man, F.; Gawne, P.J.; de Rosales, R.T.M. Nuclear Imaging of Liposomal Drug Delivery Systems: A Critical Review of Radiolabelling Methods and Applications in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, B.; Banerjee, A.; Kanekiyo, T.; Singh, J. Functionalized Liposomal Nanoparticles for Efficient Gene Delivery System to Neuronal Cell Transfection. Int. J. Pharm. 2019, 566, 717–730. [Google Scholar] [CrossRef]
- Roncato, J.F.F.; Camara, D.; Brussulo Pereira, T.C.; Quines, C.B.; Colomé, L.M.; Denardin, C.; Haas, S.; Ávila, D.S. Lipid Reducing Potential of Liposomes Loaded with Ethanolic Extract of Purple Pitanga (Eugenia Uniflora) Administered to Caenorhabditis elegans. J. Liposome Res. 2019, 29, 274–282. [Google Scholar] [CrossRef]
- Flavel, M.R.; Mechler, A.; Shahmiri, M.; Mathews, E.R.; Franks, A.E.; Chen, W.; Zanker, D.; Xian, B.; Gao, S.; Luo, J.; et al. Growth of Caenorhabditis elegans in Defined Media Is Dependent on Presence of Particulate Matter. G3 Genes Genomes Genet. 2018, 8, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Martorell, P.; Llopis, S.; Gonzalez, N.; Ramón, D.; Serrano, G.; Torrens, A.; Serrano, J.M.; Navarro, M.; Genovés, S. A Nutritional Supplement Containing Lactoferrin Stimulates the Immune System, Extends Lifespan, and Reduces Amyloid β Peptide Toxicity in Caenorhabditis elegans. Food Sci. Nutr. 2017, 5, 255–265. [Google Scholar] [CrossRef]
- Perni, M.; Aprile, F.A.; Casford, S.; Mannini, B.; Sormanni, P.; Dobson, C.M.; Vendruscolo, M. Delivery of Native Proteins into C. elegans Using a Transduction Protocol Based on Lipid Vesicles. Sci. Rep. 2017, 7, 15045. [Google Scholar] [CrossRef] [Green Version]
- Shibamura, A.; Ikeda, T.; Nishikawa, Y. A Method for Oral Administration of Hydrophilic Substances to Caenorhabditis elegans: Effects of Oral Supplementation with Antioxidants on the Nematode Lifespan. Mech. Ageing Dev. 2009, 130, 652–655. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, P.; Guerrero-Rubio, M.A.; Hernández-García, S.; Henarejos-Escudero, P.; García-Carmona, F.; Gandía-Herrero, F. Characterization of Betalain-Loaded Liposomes and Its Bioactive Potential in Vivo after Ingestion. Food Chem. 2023, 407, 135180. [Google Scholar] [CrossRef] [PubMed]
- Miyako, E.; Chechetka, S.A.; Doi, M.; Yuba, E.; Kono, K. In Vivo Remote Control of Reactions in Caenorhabditis elegans by Using Supramolecular Nanohybrids of Carbon Nanotubes and Liposomes. Angew. Chem. Int. Ed. 2015, 54, 9903–9906. [Google Scholar] [CrossRef] [PubMed]
- Esgueira, V.L.R.; Lopes, C.P.A.; dos Santos, A.C.A.; Pinto, F.; Sousa, S.A.; de Barros, D.P.C.; Leitão, J.H.; Fonseca, L.P. LipNanoCar Technology—A Versatile and Scalable Technology for the Production of Lipid Nanoparticles. In Nanotoxicology in Safety Assessment of Nanomaterials; Louro, H., Silva, M.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1357, pp. 43–82. ISBN 978-3-030-88070-5. [Google Scholar]
- Okur, N.Ü.; Çağlar, E.Ş.; Siafaka, P.I. Novel Ocular Drug Delivery Systems: An Update on Microemulsions. J. Ocul. Pharmacol. Ther. 2020, 36, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, H.H.; Felimban, R.; Almaghrabi, S.; Hasaballah, N. Nanoemulsions: Formulation, Characterization, Biological Fate, and Potential Role against COVID-19 and Other Viral Outbreaks. Colloid Interface Sci. Commun. 2021, 45, 100533. [Google Scholar] [CrossRef]
- Guo, X.; Sun, X.-T.; Liang, L.; Shi, L.-K.; Liu, R.-J.; Chang, M.; Wang, X.-G. Physical Stability, Oxidative Stability, and Bioactivity of Nanoemulsion Delivery Systems Incorporating Lipophilic Ingredients: Impact of Oil Saturation Degree. J. Agric Food Chem. 2021, 69, 5405–5415. [Google Scholar] [CrossRef]
- Zhang, L.-B.; Tang, L.; Ying, S.-H.; Feng, M.-G. Regulative Roles of Glutathione Reductase and Four Glutaredoxins in Glutathione Redox, Antioxidant Activity, and Iron Homeostasis of Beauveria Bassiana. Appl. Microbiol. Biotechnol. 2016, 100, 5907–5917. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Huang, A.Y.-T.; Kao, C.-L.; Peng, L. Poly(Amidoamine) Dendrimers: Covalent and Supramolecular Synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- Sohail, I.; Bhatti, I.A.; Ashar, A.; Sarim, F.M.; Mohsin, M.; Naveed, R.; Yasir, M.; Iqbal, M.; Nazir, A. Polyamidoamine (PAMAM) Dendrimers Synthesis, Characterization and Adsorptive Removal of Nickel Ions from Aqueous Solution. J. Mater. Res. Technol. 2020, 9, 498–506. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic MRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Walczynska, M.; Jakubowski, W.; Wasiak, T.; Kadziola, K.; Bartoszek, N.; Kotarba, S.; Siatkowska, M.; Komorowski, P.; Walkowiak, B. Toxicity of Silver Nanoparticles, Multiwalled Carbon Nanotubes, and Dendrimers Assessed with Multicellular Organism Caenorhabditis elegans. Toxicol Mech. Methods 2018, 28, 432–439. [Google Scholar] [CrossRef]
- Markowicz, J.; Uram, Ł.; Wołowiec, S.; Rode, W. Biotin Transport-Targeting Polysaccharide-Modified PAMAM G3 Dendrimer as System Delivering α-Mangostin into Cancer Cells and C. elegans Worms. Int. J. Mol. Sci. 2021, 22, 12925. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.R.; Narayan, R.J. Recent Advances in Carbon Nanomaterials for Biomedical Applications: A Review. Curr. Opin. Biomed. Eng. 2021, 17, 100262. [Google Scholar] [CrossRef]
- Hilder, T.A.; Hill, J.M. Carbon Nanotubes as Drug Delivery Nanocapsules. Curr. Appl. Phys. 2008, 8, 258–261. [Google Scholar] [CrossRef]
- Maleki, R.; Afrouzi, H.H.; Hosseini, M.; Toghraie, D.; Rostami, S. Molecular Dynamics Simulation of Doxorubicin Loading with N-Isopropyl Acrylamide Carbon Nanotube in a Drug Delivery System. Comput. Methods Programs Biomed. 2020, 184, 105303. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical Applications and Toxicities of Carbon Nanotubes. Drug Chem. Toxicol. 2022, 45, 435–450. [Google Scholar] [CrossRef]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced Biomedical Applications of Carbon Nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef]
- Lu, J.-H.; Hou, W.-C.; Tsai, M.-H.; Chang, Y.-T.; Chao, H.-R. The Impact of Background-Level Carboxylated Single-Walled Carbon Nanotubes (SWCNTs−COOH) on Induced Toxicity in Caenorhabditis elegans and Human Cells. Int. J. Environ. Res. Public Health 2022, 19, 1218. [Google Scholar] [CrossRef]
- Pacurari, M.; Qian, Y.; Fu, W.; Schwegler-Berry, D.; Ding, M.; Castranova, V.; Guo, N.L. Cell Permeability, Migration, and Reactive Oxygen Species Induced by Multiwalled Carbon Nanotubes in Human Microvascular Endothelial Cells. J. Toxicol. Environ. Health A 2012, 75, 112–128. [Google Scholar] [CrossRef] [Green Version]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical Applications of Carbon Nanomaterials: Drug and Gene Delivery Potentials. J. Cell Physiol. 2019, 234, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Eom, H.-J.; Jeong, J.-S.; Choi, J. Effect of Aspect Ratio on the Uptake and Toxicity of Hydroxylated-Multi Walled Carbon Nanotubes in the Nematode, Caenorhabditis elegans. Environ. Health Toxicol. 2015, 30, e2015001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wan, H.; Liu, Q.; Wang, D. Multi-Walled Carbon Nanotubes-Induced Alterations in MicroRNA Let-7 and Its Targets Activate a Protection Mechanism by Conferring a Developmental Timing Control. Part. Fibre Toxicol. 2017, 14, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wu, Q.; Li, Y.; Nouara, A.; Jia, R.; Wang, D. In Vivo Translocation and Toxicity of Multi-Walled Carbon Nanotubes Are Regulated by MicroRNAs. Nanoscale 2014, 6, 4275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jin, L.; Wang, Y.; Kong, Y.; Wang, D. Prolonged Exposure to Multi-Walled Carbon Nanotubes Dysregulates Intestinal Mir-35 and Its Direct Target MAB-3 in Nematode Caenorhabditis elegans. Sci. Rep. 2019, 9, 12144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, N.; Yang, J.; Kim, H.-M.; Jo, E.; Kim, P.-J.; Choi, K.; Choi, J. Potential Toxicity of Differential Functionalized Multiwalled Carbon Nanotubes (MWCNT) in Human Cell Line (BEAS2B) and Caenorhabditis elegans. J. Toxicol. Environ. Health A 2014, 77, 1399–1408. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Wang, D. A MicroRNA-Mediated Insulin Signaling Pathway Regulates the Toxicity of Multi-Walled Carbon Nanotubes in Nematode Caenorhabditis elegans. Sci. Rep. 2016, 6, 23234. [Google Scholar] [CrossRef] [Green Version]
- Dinc, B.; Sen, E. Toxicity of Short Multi-Walled Carbon Nanotubes in Caenorhabditis elegans. Fuller. Nanotub. Carbon Nanostructures 2022, 30, 646–656. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon Nanotubes: Evaluation of Toxicity at Biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Vijayalakshmi, V.; Sadanandan, B.; Venkataramanaiah Raghu, A. Single Walled Carbon Nanotubes in High Concentrations Is Cytotoxic to the Human Neuronal Cell LN18. Results Chem. 2022, 4, 100484. [Google Scholar] [CrossRef]
- Chen, P.-H.; Hsiao, K.-M.; Chou, C.-C. Molecular Characterization of Toxicity Mechanism of Single-Walled Carbon Nanotubes. Biomaterials 2013, 34, 5661–5669. [Google Scholar] [CrossRef]
- Goodwin, C.M.; Lewis, G.G.; Fiorella, A.; Ellison, M.D.; Kohn, R. Synthesis and Toxicity Testing of Cysteine-Functionalized Single-Walled Carbon Nanotubes with Caenorhabditis elegans. RSC Adv. 2014, 4, 5893. [Google Scholar] [CrossRef]
- Hendler-Neumark, A.; Wulf, V.; Bisker, G. In Vivo Imaging of Fluorescent Single-Walled Carbon Nanotubes within C. elegans Nematodes in the near-Infrared Window. Mater. Today Bio 2021, 12, 100175. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene Polymers: A Brief Review. C J. Carbon Res. 2020, 6, 71. [Google Scholar] [CrossRef]
- Cha, Y.J.; Lee, J.; Choi, S.S. Apoptosis-Mediated in Vivo Toxicity of Hydroxylated Fullerene Nanoparticles in Soil Nematode Caenorhabditis elegans. Chemosphere 2012, 87, 49–54. [Google Scholar] [CrossRef]
- Cong, W.; Wang, P.; Qu, Y.; Tang, J.; Bai, R.; Zhao, Y.; Chunying, C.; Bi, X. Evaluation of the Influence of Fullerenol on Aging and Stress Resistance Using Caenorhabditis elegans. Biomaterials 2015, 42, 78–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Shakoor, S.; Gong, J.R.; Wang, D. Transgenerational Safety of Nitrogen-Doped Graphene Quantum Dots and the Underlying Cellular Mechanism in Caenorhabditis elegans. Toxicol. Res. 2015, 4, 270–280. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Zhang, X.; Cheng, J.; Zhang, J.; Chen, M.; Wu, T. A Deep Learning Analysis Reveals Nitrogen-Doped Graphene Quantum Dots Damage Neurons of Nematode Caenorhabditis elegans. Nanomaterials 2021, 11, 3314. [Google Scholar] [CrossRef]
- Nigamatzyanova, L.; Fakhrullin, R. Dark-Field Hyperspectral Microscopy for Label-Free Microplastics and Nanoplastics Detection and Identification in Vivo: A Caenorhabditis elegans Study. Environ. Pollut. 2021, 271, 116337. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Q.; Wang, D. An Epigenetic Signal Encoded Protection Mechanism Is Activated by Graphene Oxide to Inhibit Its Induced Reproductive Toxicity in Caenorhabditis elegans. Biomaterials 2016, 79, 15–24. [Google Scholar] [CrossRef]
- Dou, T.; Chen, J.; Wang, R.; Pu, X.; Wu, H.; Zhao, Y. Complementary Protective Effects of Autophagy and Oxidative Response against Graphene Oxide Toxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2022, 248, 114289. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, J.; Yang, J.; Joo, S.-W.; Hong, J.; Choi, J. Graphene Oxide Nano-Bio Interaction Induces Inhibition of Spermatogenesis and Disturbance of Fatty Acid Metabolism in the Nematode Caenorhabditis elegans. Toxicology 2018, 410, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, J.; Rui, Q.; Wang, D. Long-Term Exposure to Thiolated Graphene Oxide in the Range of Μg/L Induces Toxicity in Nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 616, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, N.; Rajput, V.D.; Mandzhieva, S.; Minkina, T.; Saharan, B.S.; Kumar, D.; Sadh, P.K.; Duhan, J.S. Advances in Biopolymeric Nanopesticides: A New Eco-Friendly/Eco-Protective Perspective in Precision Agriculture. Nanomaterials 2022, 12, 3964. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; el Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide Research: Current Trends and Future Priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Guleria, G.; Thakur, S.; Shandilya, M.; Sharma, S.; Thakur, S.; Kalia, S. Nanotechnology for Sustainable Agro-Food Systems: The Need and Role of Nanoparticles in Protecting Plants and Improving Crop Productivity. Plant Physiol. Biochem. 2023, 194, 533–549. [Google Scholar] [CrossRef]
- Arora, S.; Murmu, G.; Mukherjee, K.; Saha, S.; Maity, D. A Comprehensive Overview of Nanotechnology in Sustainable Agriculture. J. Biotechnol. 2022, 355, 21–41. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, L.; Zhang, L.; Shen, X.; Kong, L.; Wu, T. Research Advances on the Adverse Effects of Nanomaterials in a Model Organism, Caenorhabditis elegans. Environ. Toxicol. Chem. 2021, 40, 2406–2424. [Google Scholar] [CrossRef]
- Helmcke, K.J.; Avila, D.S.; Aschner, M. Utility of Caenorhabditis elegans in High Throughput Neurotoxicological Research. Neurotoxicol. Teratol. 2010, 32, 62–67. [Google Scholar] [CrossRef]
- Jacques, M.T.; Oliveira, J.L.; Campos, E.V.R.; Fraceto, L.F.; Ávila, D.S. Safety Assessment of Nanopesticides Using the Roundworm Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2017, 139, 245–253. [Google Scholar] [CrossRef]
- Sanches Moraes, B.K.; Vieira, S.M.; Salgueiro, W.G.; Michels, L.R.; Colomé, L.M.; Avila, D.S.; Haas, S.E. Clozapine-Loaded Polysorbate-Coated Polymeric Nanocapsules: Physico-Chemical Characterization and Toxicity Evaluation in Caenorhabditis elegans Model. J. Nanosci. Nanotechnol. 2016, 16, 1257–1264. [Google Scholar] [CrossRef]
- Charão, M.; Souto, C.; Brucker, N.; Barth, A.; Jornada, D.; Fagundes, D.; Ávila, D.; Eifler-Lima, V.; Guterres, S.; Pohlmann, A.; et al. Caenorhabditis elegans as an Alternative in Vivo Model to Determine Oral Uptake, Nanotoxicity, and Efficacy of Melatonin-Loaded Lipid-Core Nanocapsules on Paraquat Damage. Int. J. Nanomed. 2015, 10, 5093–5106. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B.B. Neem (Azadirachta Indica): An Indian Traditional Panacea with Modern Molecular Basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Pascoli, M.; de Lima, R.; Fraceto, L.F. Neem Oil and Crop Protection: From Now to the Future. Front. Plant Sci. 2016, 7, e1494. [Google Scholar] [CrossRef] [Green Version]
- Pascoli, M.; Jacques, M.T.; Agarrayua, D.A.; Avila, D.S.; Lima, R.; Fraceto, L.F. Neem Oil Based Nanopesticide as an Environmentally-Friendly Formulation for Applications in Sustainable Agriculture: An Ecotoxicological Perspective. Sci. Total Environ. 2019, 677, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, N.R.; Roncato, J.F.F.; Pascoli, M.; e Sousa, J.M.F.; Windberg, L.F.; Rossatto, F.C.P.; de Jesus Soares, J.; Denardin, E.L.G.; Puntel, R.L.; Zimmer, K.R.; et al. Clove Oil-Loaded Zein Nanoparticles as Potential Bioinsecticide Agent with Low Toxicity. Sustain. Chem. Pharm. 2021, 24, 100554. [Google Scholar] [CrossRef]
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; Kershaw, P.J., Turra, A., Galgani, F., Eds.; GESAMP Report and Studies No. 99; IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019. [Google Scholar]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; el Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- European Commission; Joint Research Centre; Institute for Health; Protection, C; European Commission; Institute for Reference Materials, Measurements; Institute for Environment. Sustainability Considerations on a Definition of Nanomaterial for Regulatory Purposes; Publications Office: Luxembourg, 2010. [Google Scholar]
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential Health Impact of Environmentally Released Micro- and Nanoplastics in the Human Food Production Chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, W.; Tan, Q.; Xiao, Y.; Shi, Y.; Lei, J.; Li, Z.; Hou, Y.; Liu, T.; Li, Y. The Toxic Differentiation of Micro- and Nanoplastics Verified by Gene-Edited Fluorescent Caenorhabditis elegans. Sci. Total Environ. 2023, 856, 159058. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and Toxicity: A Preliminary Study of Effects of Nanoplastic Particles on Human Lung Epithelial Cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; de Falco, M.; Laforgia, V.; Valiante, S. Polystyrene Nanoparticles Internalization in Human Gastric Adenocarcinoma Cells. Toxicol. Vitr. 2016, 31, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Petri-Fink, A.; Rothen-Rutishauser, B. Nanoplastic Impact on Human Health—A 3D Intestinal Model to Study the Interaction with Nanoplastic Particles. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Cocca, M., di Pace, E., Errico, M.E., Gentile, G., Montarsolo, A., Mossotti, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 167–170. ISBN1 978-3-319-71278-9. ISBN2 978-3-319-71279-6. [Google Scholar]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Stapleton, P.A. Toxicological Considerations of Nano-Sized Plastics. AIMS Environ. Sci. 2019, 6, 367–378. [Google Scholar] [CrossRef]
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.-A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an Ex Vivo Human Placental Perfusion Model. Environ. Health Perspect. 2015, 123, 1280–1286. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.; Wagner, M. Characterisation of Nanoplastics during the Degradation of Polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.K.; Hong, S.H.; Eo, S.; Han, G.M.; Shim, W.J. Rapid Production of Micro- and Nanoplastics by Fragmentation of Expanded Polystyrene Exposed to Sunlight. Environ. Sci Technol. 2020, 54, 11191–11200. [Google Scholar] [CrossRef]
- Piccardo, M.; Renzi, M.; Terlizzi, A. Nanoplastics in the Oceans: Theory, Experimental Evidence and Real World. Mar. Pollut. Bull. 2020, 157, 111317. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption Behavior of Organic Pollutants and Metals on Micro/Nanoplastics in the Aquatic Environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Huang, C.-W.; Yen, P.-L.; Kuo, Y.-H.; Chang, C.-H.; Liao, V.H.-C. Nanoplastic Exposure in Soil Compromises the Energy Budget of the Soil Nematode C. elegans and Decreases Reproductive Fitness. Environ. Pollut. 2022, 312, 120071. [Google Scholar] [CrossRef]
- Qu, M.; Chen, H.; Lai, H.; Liu, X.; Wang, D.; Zhang, X. Exposure to Nanopolystyrene and Its 4 Chemically Modified Derivatives at Predicted Environmental Concentrations Causes Differently Regulatory Mechanisms in Nematode Caenorhabditis elegans. Chemosphere 2022, 305, 135498. [Google Scholar] [CrossRef]
- Zhao, Y.; Hua, X.; Bian, Q.; Wang, D. Nanoplastic Exposure at Predicted Environmental Concentrations Induces Activation of Germline Ephrin Signal Associated with Toxicity Formation in the Caenorhabditis elegans Offspring. Toxics 2022, 10, 699. [Google Scholar] [CrossRef]
- Jewett, E.; Arnott, G.; Connolly, L.; Vasudevan, N.; Kevei, E. Microplastics and Their Impact on Reproduction—Can We Learn From the C. elegans Model? Front. Toxicol. 2022, 4, 748912. [Google Scholar] [CrossRef]
- Mueller, M.-T.; Fueser, H.; Trac, L.N.; Mayer, P.; Traunspurger, W.; Höss, S. Surface-Related Toxicity of Polystyrene Beads to Nematodes and the Role of Food Availability. Environ. Sci. Technol. 2020, 54, 1790–1798. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene Nanoparticles: Sources, Occurrence in the Environment, Distribution in Tissues, Accumulation and Toxicity to Various Organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; Rapra Review Reports; Rapra Technology Ltd: Shawbury, UK, 2000; ISBN 978-1-85957-191-0. [Google Scholar]
- Qiu, Y.; Liu, Y.; Li, Y.; Li, G.; Wang, D. Effect of Chronic Exposure to Nanopolystyrene on Nematode Caenorhabditis elegans. Chemosphere 2020, 256, 127172. [Google Scholar] [CrossRef]
- Qiu, Y.; Luo, L.; Yang, Y.; Kong, Y.; Li, Y.; Wang, D. Potential Toxicity of Nanopolystyrene on Lifespan and Aging Process of Nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 705, 135918. [Google Scholar] [CrossRef]
- Winbush, A.; Gruner, M.; Hennig, G.W.; van der Linden, A.M. Long-Term Imaging of Circadian Locomotor Rhythms of a Freely Crawling C. elegans Population. J. Neurosci. Methods 2015, 249, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-W.; Wu, Y.-C.; Liao, V.H.-C. Early Developmental Nanoplastics Exposure Disturbs Circadian Rhythms Associated with Stress Resistance Decline and Modulated by DAF-16 and PRDX-2 in C. elegans. J. Hazard. Mater. 2022, 423, 127091. [Google Scholar] [CrossRef]
- Huang, G.; Ma, Y.; Xie, D.; Zhao, C.; Zhu, L.; Xie, G.; Wu, P.; Wang, W.; Zhao, Z.; Cai, Z. Evaluation of Nanoplastics Toxicity in the Soil Nematode Caenorhabditis elegans by ITRAQ-Based Quantitative Proteomics. Sci. Total Environ. 2023, 862, 160646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, C.; Li, M.; Ke, J.; Huang, Y.; Bian, Y.; Guo, S.; Wu, Y.; Han, Y.; Liu, M. Neurodevelopmental Toxicity of Polystyrene Nanoplastics in Caenorhabditis elegans and the Regulating Effect of Presenilin. ACS Omega 2020, 5, 33170–33177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Qu, M.; Wang, D. Response of Tyramine and Glutamate Related Signals to Nanoplastic Exposure in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2021, 217, 112239. [Google Scholar] [CrossRef]
- Liu, H.; Tian, L.; Qu, M.; Wang, D. Acetylation Regulation Associated with the Induction of Protective Response to Polystyrene Nanoparticles in Caenorhabditis elegans. J. Hazard. Mater. 2021, 411, 125035. [Google Scholar] [CrossRef] [PubMed]
- Skvortsova, K.; Iovino, N.; Bogdanović, O. Functions and Mechanisms of Epigenetic Inheritance in Animals. Nat. Rev. Mol. Cell. Biol. 2018, 19, 774–790. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, R.; Wang, D. Induction of Protective Response to Polystyrene Nanoparticles Associated with Methylation Regulation in Caenorhabditis elegans. Chemosphere 2021, 271, 129589. [Google Scholar] [CrossRef]
- Yu, C.-W.; Luk, T.C.; Liao, V.H.-C. Long-Term Nanoplastics Exposure Results in Multi and Trans-Generational Reproduction Decline Associated with Germline Toxicity and Epigenetic Regulation in Caenorhabditis elegans. J. Hazard. Mater. 2021, 412, 125173. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Tian, L.; Wang, S.; Wang, D. Size-Dependent Transgenerational Toxicity Induced by Nanoplastics in Nematode Caenorhabditis elegans. Sci. Total Environ. 2021, 790, 148217. [Google Scholar] [CrossRef]
- Xu, R.; Hua, X.; Rui, Q.; Wang, D. Alteration in Wnt Signaling Mediates Induction of Transgenerational Toxicity of Polystyrene Nanoplastics in C. elegans. NanoImpact 2022, 28, 100425. [Google Scholar] [CrossRef]
- Liu, H.; Wang, D. Intestinal Mitochondrial Unfolded Protein Response Induced by Nanoplastic Particles in Caenorhabditis elegans. Chemosphere 2021, 267, 128917. [Google Scholar] [CrossRef]
- Hua, X.; Zhao, Y.; Yuan, Y.; Zhang, L.; Bian, Q.; Wang, D. Nanoplastics Cause Transgenerational Toxicity through Inhibiting Germline MicroRNA Mir-38 in C. elegans. J. Hazard. Mater. 2022, 437, 129302. [Google Scholar] [CrossRef]

| Nanoparticles | Size Range (nm) | Applications |
|---|---|---|
| Silver | 2–20 | Antibacterial activity |
| Gold (spheres and rods) | 2–150 | Site-specific imaging and hyperthermia |
| Titanium dioxide | 5–50 | Ultraviolet radiation protection (sunscreen transparent on skin) |
| Iron oxide | 10–250 | MRI, site-specific imaging, and magnetic hyperthermia |
| Silica (mesoposous) | 20–300 | Drug delivery and tumor targeting |
| Carbon (fullerenes and nanotubes) | 1–40 | MRI contrast and antioxidants |
| Quantum dots (core/shell) | 2–20 | Fluorescence imaging |
| Copolymers agents | <200 | Drug and gene delivery |
| Dendrimer-based conjugates | 2–50 | Drug delivery and targeting |
| Exosomes (vesicles) | 30–90 | Regenerative therapy |
| Liposomes | 50–350 | Drug and gene delivery |
| Metabolic Pathways | No. of Active Genes | PAMAM |
|---|---|---|
| Cytoplasmic ribossomal proteins | 63 | rpl-6 (3.11) rpl-10 (3.11) rpl-13 (−4.93) rpl-15 (9.31) rpl-17 (7.31) rpl-18 (−12.67) rpl-26 (2.87) rpl-32 (−2.74) rpl-43 (−7.90) rps-4 (2.25) rps-5 (5.42) rps-9 (−3.38) rps-12(2.12) rps-13 (−5.57) rps-14 (1.04) rps-17 (2.06) rps-30 (−2.98) |
| Glycolysis | 25 | hxk-1 (3.07) acl-4 (−6.67) fbp-1 (−3.67) aldo-2 (4.10) pyc-1 (3.07) pck-1 (2.27) |
| DNA replication | 25 | orc-1 (−4.99) mcm-3 (3.17) mcm-2 (−18.54) arpa (2.06) rfc-1 (−1.04) f10c2.4 (2.69) cdc-6 (2.04) |
| LIN-12-Notch lateral signaling | 15 | let-23 (2.04) lst-2 (2.89) dpy-23 (2.19) mpk-1 (2.79) unc-101 (2.02) |
| Sex determination | 17 | sex-1 (−2.89) sea-1 (7.70) sdc-3 (1.04) fem-1 (3.00) sel-10 (2.03) |
| Translation factors | 31 | eif-3.H (−2.08) eif-3.G (−5.72) ife-3 (2.67) eef-1g (−2.13) eft-2 (−7.23) eif-2α (−2.03) eif-3b (2.20) |
| Total number of genes (+ upregulated, − downregulated) | 47 (+29, −18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubert, P.; Gubert, G.; Oliveira, R.C.d.; Fernandes, I.C.O.; Bezerra, I.C.; Ramos, B.d.; Lima, M.F.d.; Rodrigues, D.T.; Cruz, A.F.N.d.; Pereira, E.C.; et al. Caenorhabditis elegans as a Prediction Platform for Nanotechnology-Based Strategies: Insights on Analytical Challenges. Toxics 2023, 11, 239. https://doi.org/10.3390/toxics11030239
Gubert P, Gubert G, Oliveira RCd, Fernandes ICO, Bezerra IC, Ramos Bd, Lima MFd, Rodrigues DT, Cruz AFNd, Pereira EC, et al. Caenorhabditis elegans as a Prediction Platform for Nanotechnology-Based Strategies: Insights on Analytical Challenges. Toxics. 2023; 11(3):239. https://doi.org/10.3390/toxics11030239
Chicago/Turabian StyleGubert, Priscila, Greici Gubert, Ronei Cardoso de Oliveira, Isabel Cristina Oliveira Fernandes, Iverson Conrado Bezerra, Bruna de Ramos, Milena Ferreira de Lima, Daniela Teixeira Rodrigues, Adriana Farias Nunes da Cruz, Ernesto Chaves Pereira, and et al. 2023. "Caenorhabditis elegans as a Prediction Platform for Nanotechnology-Based Strategies: Insights on Analytical Challenges" Toxics 11, no. 3: 239. https://doi.org/10.3390/toxics11030239
APA StyleGubert, P., Gubert, G., Oliveira, R. C. d., Fernandes, I. C. O., Bezerra, I. C., Ramos, B. d., Lima, M. F. d., Rodrigues, D. T., Cruz, A. F. N. d., Pereira, E. C., Ávila, D. S., & Mosca, D. H. (2023). Caenorhabditis elegans as a Prediction Platform for Nanotechnology-Based Strategies: Insights on Analytical Challenges. Toxics, 11(3), 239. https://doi.org/10.3390/toxics11030239