Imidacloprid and Its Bioactive Metabolite, Desnitro-Imidacloprid, Differentially Affect Ovarian Antral Follicle Growth, Morphology, and Hormone Synthesis In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
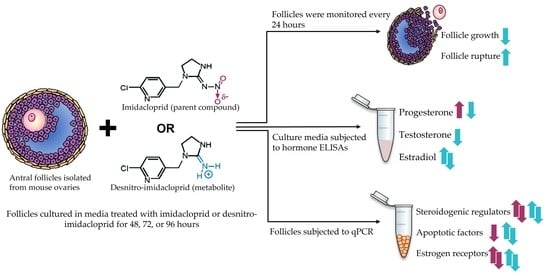
2.3. Antral Follicle Culture
2.4. Antral Follicle Growth and Rupture
2.5. Steroid Hormone Quantification
2.6. Gene Expression
2.7. Statistical Analyses
3. Results
3.1. Effects of IMI and DNI on Antral Follicle Growth and Follicle Rupture
3.2. Effects of IMI and DNI on Sex Steroid Hormone Levels
3.3. Effects of IMI and DNI on Gene Expression
3.3.1. Steroidogenic Regulators
3.3.2. Estrogen Receptors
3.3.3. Apoptotic Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ospina, M.; Wong, L.-Y.; Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Calafat, A.M. Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015–2016 national health and nutrition examination survey. Environ. Res. 2019, 176, 108555. [Google Scholar] [CrossRef] [PubMed]
- Taillebois, E.; Cartereau, A.; Jones, A.K.; Thany, S.H. Neonicotinoid insecticides mode of action on insect nicotinic acetylcholine receptors using binding studies. Pestic. Biochem. Physiol. 2018, 151, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Hitaj, C.; Smith, D.J.; Code, A.; Wechsler, S.; Esker, P.D.; Douglas, M.R. Sowing Uncertainty: What We Do and Don’t Know about the Planting of Pesticide-Treated Seed. Bioscience 2020, 70, 390–403. [Google Scholar] [CrossRef] [Green Version]
- Smalling, K.L.; Hladik, M.L.; Sanders, C.J.; Kuivila, K.M. Leaching and sorption of neonicotinoid insecticides and fungicides from seed coatings. J. Environ. Sci. Health Part B 2017, 53, 176–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Chang, C.; Lou, J.; Zhao, M.; Lu, C. Potential human exposures to neonicotinoid insecticides: A review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef]
- Sultana, T.; Murray, C.; Kleywegt, S.; Metcalfe, C.D. Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere 2018, 202, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Han, Q.; Wang, Y.; He, Z. Five degradates of imidacloprid in source water, treated water, and tap water in Wuhan, central China. Sci. Total Environ. 2020, 741, 140227. [Google Scholar] [CrossRef] [PubMed]
- Mahai, G.; Wan, Y.; Xia, W.; Wang, A.; Shi, L.; Qian, X.; He, Z.; Xu, S. A nationwide study of occurrence and exposure assessment of neonicotinoid insecticides and their metabolites in drinking water of China. Water Res. 2021, 189, 116630. [Google Scholar] [CrossRef]
- Mahai, G.; Wan, Y.; Xia, W.; Yang, S.; He, Z.; Xu, S. Neonicotinoid insecticides in surface water from the central Yangtze River, China. Chemosphere 2019, 229, 452–460. [Google Scholar] [CrossRef]
- Fuentes, E.; Cid, C.; Báez, M.E. Determination of imidacloprid in water samples via photochemically induced fluorescence and second-order multivariate calibration. Talanta 2015, 134, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Hruby, C.E.; Vargo, J.D.; Field, R.W. Occurrence of neonicotinoids and sulfoxaflor in major aquifer groups in Iowa. Chemosphere 2021, 281, 130856. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Jander, D.A.; Leimkuehler, W.M.; Casida, J.E. Neonicotinoid Insecticides: Reduction and Cleavage of Imidacloprid Nitroimine Substituent by Liver Microsomal and Cytosolic Enzymes. Chem. Res. Toxicol. 2002, 15, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.K.; Webb, D.T.; Nagorzanski, M.R.; Kolpin, D.W.; Hladik, M.L.; Cwiertny, D.M.; Lefevre, G.H. Chlorinated Byproducts of Neonicotinoids and Their Metabolites: An Unrecognized Human Exposure Potential? Environ. Sci. Technol. Lett. 2019, 6, 98–105. [Google Scholar] [CrossRef]
- Brunet, J.-L.; Maresca, M.; Fantini, J.; Belzunces, L.P. Human intestinal absorption of imidacloprid with Caco-2 cells as enterocyte model. Toxicol. Appl. Pharmacol. 2003, 194, 1–9. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of Selective Action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Loser, D.; Grillberger, K.; Hinojosa, M.G.; Blum, J.; Haufe, Y.; Danker, T.; Johansson, Y.; Möller, C.; Nicke, A.; Bennekou, S.H.; et al. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 2021, 95, 3695–3716. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Imidacloprid, Thiacloprid, and Their Imine Derivatives Up-Regulate the α4β2 Nicotinic Acetylcholine Receptor in M10 Cells. Toxicol. Appl. Pharmacol. 2000, 169, 114–120. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Minor structural changes in nicotinoid insecticides confer differential subtype selectivity for mammalian nicotinic acetylcholine receptors. Br. J. Pharmacol. 1999, 127, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, U.; Srivastava, M.; Trivedi, P.; Garg, V.; Srivastava, L. Disposition and acute toxicity of imidacloprid in female rats after single exposure. Food Chem. Toxicol. 2014, 68, 190–195. [Google Scholar] [CrossRef]
- Guraya, S.S. Recent Advances in the Morphology, Histochemistry, and Biochemistry of the Developing Mammalian Ovary. Int. Rev. Cytol. 1977, 51, 49–131. [Google Scholar] [CrossRef] [PubMed]
- Kaprara, A.; Huhtaniemi, I.T. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism 2018, 86, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, S.; Cohen-Tannoudji, J.; Guigon, C.J. Estradiol Signaling at the Heart of Folliculogenesis: Its Potential Deregulation in Human Ovarian Pathologies. Int. J. Mol. Sci. 2022, 23, 512. [Google Scholar] [CrossRef]
- Greenfeld, C.R.; Babus, J.K.; Furth, P.A.; Marion, S.; Hoyer, P.B.; Flaws, J.A. BAX is involved in regulating follicular growth, but is dispensable for follicle atresia in adult mouse ovaries. Reproduction 2007, 133, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Flaws, J.A.; Hirshfield, A.N.; Hewitt, J.A.; Babus, J.K.; Furth, P.A. Effect of Bcl-2 on the Primordial Follicle Endowment in the Mouse Ovary1. Biol. Reprod. 2001, 64, 1153–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Peretz, J.; Pan, Y.-X.; Helferich, W.G.; Flaws, J.A. Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles. Toxicol. Appl. Pharmacol. 2016, 293, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Paulose, T.; Hernández-Ochoa, I.; Basavarajappa, M.S.; Peretz, J.; Flaws, J.A. Increased Sensitivity of Estrogen Receptor Alpha Overexpressing Antral Follicles to Methoxychlor and Its Metabolites. Toxicol. Sci. 2011, 120, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Hannon, P.R.; Brannick, K.E.; Wang, W.; Gupta, R.K.; Flaws, J.A. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2015, 284, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Meling, D.D.; Warner, G.R.; Szumski, J.R.; Gao, L.; Gonsioroski, A.V.; Rattan, S.; Flaws, J.A. The effects of a phthalate metabolite mixture on antral follicle growth and sex steroid synthesis in mice. Toxicol. Appl. Pharmacol. 2019, 388, 114875. [Google Scholar] [CrossRef]
- Gonsioroski, A.; Meling, D.D.; Gao, L.; Plewa, M.J.; Flaws, J.A. Iodoacetic acid inhibits follicle growth and alters expression of genes that regulate apoptosis, the cell cycle, estrogen receptors, and ovarian steroidogenesis in mouse ovarian follicles. Reprod. Toxicol. 2019, 91, 101–108. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.; Srivastava, L. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem. Toxicol. 2011, 49, 3086–3089. [Google Scholar] [CrossRef]
- Khera, P.V.A.K.S. Effect of Imidacloprid on Reproduction of Female Albino Rats in Three Generation Study. J. Vet. Sci. Technol. 2016, 7, 4. [Google Scholar] [CrossRef]
- Uroz, F.J.; Arrebola, F.J.; Egea-González, F.J.; Martínez-Vidal, J.L. Monitoring of 6-chloronicotinic acid in human urine by gas chromatography-tandem mass spectrometry as indicator of exposure to the pesticide imidacloprid. Analyst 2001, 126, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Kavvalakis, M.P.; Tzatzarakis, M.N.; Theodoropoulou, E.P.; Barbounis, E.G.; Tsakalof, A.K.; Tsatsakis, A.M. Development and application of LC–APCI–MS method for biomonitoring of animal and human exposure to imidacloprid. Chemosphere 2013, 93, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Ueyama, J.; Kondo, T.; Saito, I.; Murata, K.; Iwata, T.; Wakusawa, S.; Kamijima, M. Quantitation of neonicotinoid metabolites in human urine using GC-MS. J. Chromatogr. B 2013, 941, 109–115. [Google Scholar] [CrossRef]
- Harada, K.H.; Tanaka, K.; Sakamoto, H.; Imanaka, M.; Niisoe, T.; Hitomi, T.; Kobayashi, H.; Okuda, H.; Inoue, S.; Kusakawa, K.; et al. Biological Monitoring of Human Exposure to Neonicotinoids Using Urine Samples, and Neonicotinoid Excretion Kinetics. PLoS ONE 2016, 11, e0146335. [Google Scholar] [CrossRef] [Green Version]
- Osaka, A.; Ueyama, J.; Kondo, T.; Nomura, H.; Sugiura, Y.; Saito, I.; Nakane, K.; Takaishi, A.; Ogi, H.; Wakusawa, S.; et al. Exposure characterization of three major insecticide lines in urine of young children in Japan—Neonicotinoids, organophosphates, and pyrethroids. Environ. Res. 2016, 147, 89–96. [Google Scholar] [CrossRef]
- Basavarajappa, M.S.; Karman, B.N.; Wang, W.; Gupta, R.K.; Flaws, J.A. Methoxychlor induces atresia by altering Bcl2 factors and inducing caspase activity in mouse ovarian antral follicles in vitro. Reprod. Toxicol. 2012, 34, 545–551. [Google Scholar] [CrossRef] [Green Version]











| Gene Name | Symbol | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| Beta actin | βactin | GGGCACAGTGTGGGTGAC | CTGGCACCACACCTTCTAC |
| Steroidogenic acute regulatory protein | Star | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG |
| Cytochrome P450 cholesterol side-chain cleavage | Cyp11a1 | AGATCCCTTCCCCTGGTGACAATG | CGCATGAGAAGAGTATCGACGCATC |
| Cytochrome P450 steroid 17-a-hydroxylase 1 | Cyp17a1 | CCAGGACCCAAGTGTGTTCT | CCTGATACGAAGCACTTCTCG |
| Cytochrome P450 aromatase | Cyp19a1 | CATGGTCCCGGAAACTGTGA | GTAGTAGTTGCAGGCACTTC |
| 3b-Hydroxysteroid dehydrogenase 1 | Hsd3b1 | CAGGAGAAAGAACTGCAGGAGGTC | GCACACTTGCTTGAACACAGGC |
| 17b-Hydroxysteroid dehydrogenase 1 | Hsd17b1 | AAGCGGTTCGTGGAGAAGTAG | ACTGTGCCAGCAAGTTTGCG |
| Estrogen receptor 1 (alpha) | Esr1 | AATTCTGACAATCGACGCCAG | GTGCTTCAACATTCTCCCTCCTC |
| Estrogen receptor 2 (beta) | Esr2 | GGAATCTCTTCCCAGCAGCA | GGGACCACATTTTTGCACTT |
| B cell lymphoma 2 | Bcl2 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC |
| Bcl2-associated X protein | Bax | TGAAGACAGGGGCCTTTTTG | AATTCGCCGGAGACACTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourikes, V.E.; Santacruz Márquez, R.; Deviney, A.; Neff, A.M.; Laws, M.J.; Flaws, J.A. Imidacloprid and Its Bioactive Metabolite, Desnitro-Imidacloprid, Differentially Affect Ovarian Antral Follicle Growth, Morphology, and Hormone Synthesis In Vitro. Toxics 2023, 11, 349. https://doi.org/10.3390/toxics11040349
Mourikes VE, Santacruz Márquez R, Deviney A, Neff AM, Laws MJ, Flaws JA. Imidacloprid and Its Bioactive Metabolite, Desnitro-Imidacloprid, Differentially Affect Ovarian Antral Follicle Growth, Morphology, and Hormone Synthesis In Vitro. Toxics. 2023; 11(4):349. https://doi.org/10.3390/toxics11040349
Chicago/Turabian StyleMourikes, Vasiliki E., Ramsés Santacruz Márquez, Ashley Deviney, Alison M. Neff, Mary J. Laws, and Jodi A. Flaws. 2023. "Imidacloprid and Its Bioactive Metabolite, Desnitro-Imidacloprid, Differentially Affect Ovarian Antral Follicle Growth, Morphology, and Hormone Synthesis In Vitro" Toxics 11, no. 4: 349. https://doi.org/10.3390/toxics11040349
APA StyleMourikes, V. E., Santacruz Márquez, R., Deviney, A., Neff, A. M., Laws, M. J., & Flaws, J. A. (2023). Imidacloprid and Its Bioactive Metabolite, Desnitro-Imidacloprid, Differentially Affect Ovarian Antral Follicle Growth, Morphology, and Hormone Synthesis In Vitro. Toxics, 11(4), 349. https://doi.org/10.3390/toxics11040349