One Health Approach to Tackle Microbial Contamination on Poultries—A Systematic Review
Abstract
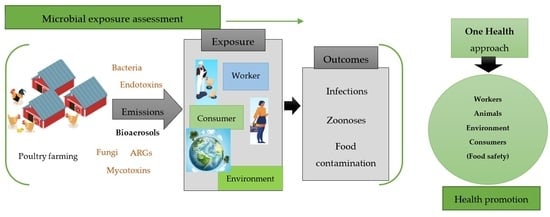
:1. Introduction
2. Materials and Methods
2.1. Registration
2.2. Search Strategy, Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
3. Results
Characteristics of the Selected Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPBES. Workshop Report on Biodiversity and Pandemics of the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES); IPBES Secretariat: Bonn, Germany, 2020. [Google Scholar] [CrossRef]
- Viegas, C.; Moniz, G.; Pargana, J.; Marques, S.; Resende, C.; Martins, C.; Arez, A.P.; Ceratto, N.; Viegas, S. Biodiversity and health: Investing in biodiversity protection towards health gains. In From Life Molecules to Global Health; Principia Editora: Parede, Portugal, 2021; Available online: https://principia.pt/livro/from-life-molecules-to-global-health/ (accessed on 23 January 2023).
- Sinclair, J.R. Importance of a One Health approach in advancing global health security and the Sustainable Development Goals. Rev. Sci. Tech. l’OIE 2019, 38, 145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Patyal, A. Impacts of intensive poultry farming on “one health” in developing countries: Challenges and remedies. Explor. Anim. Med. Res. 2020, 10, 100–111. [Google Scholar]
- Smit, L.A.M.; Heederik, D. Impacts of Intensive Livestock Production on Human Health in Densely Populated Regions. GeoHealth 2017, 1, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Xiao, X.; Robinson, T.P. Intensifying poultry production systems and the emergence of avian influenza in China: A ‘One Health/Ecohealth’ epitome. Arch. Public Health 2017, 75, 48. [Google Scholar] [CrossRef] [PubMed]
- Alizon, S.; Hurford, A.; Mideo, N.; Van Baalen, M. Virulence evolution and the trade-off hypothesis: History, current state of affairs and the future. J. Evol. Biol. 2009, 22, 245–259. [Google Scholar] [CrossRef]
- Cruciani, D.; Crotti, S.; Maresca, C.; Pecorelli, I.; Verdini, E.; Rodolfi, M.; Scoccia, E.; Spina, S.; Valentini, A.; Agnetti, F. Preliminary Investigation about Aspergillus spp. Spread in Umbrian Avian Farms. J. Fungi 2022, 8, 1213. [Google Scholar] [CrossRef]
- Layton, D.S.; Choudhary, A.; Bean, A.G.D. Breaking the chain of zoonoses through biosecurity in livestock. Vaccine 2017, 35, 5967–5973. [Google Scholar] [CrossRef]
- Douphrate, D.I. Animal Agriculture and the One Health Approach. J. Agromed. 2021, 26, 85–87. [Google Scholar] [CrossRef]
- FAO. Industrial Livestock Production and Global Health Risks: Pro-Poor Livestock Policy Initiative: A Living from Livestock; Pro-Poor Livestock Policy Initiative (PPLPI) Research Report; FAO: Rome, Italy, 2007. [Google Scholar]
- Sabino, R.; Faísca, V.M.; Carolino, E.; Veríssimo, C.; Viegas, C. Occupational exposure to Aspergillus by swine and poultry farm workers in Portugal. J. Toxicol. Environ. Health Part A 2012, 75, 1381–1391. [Google Scholar] [CrossRef]
- Codex Alimentarius Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for Its Application. 2003. Available online: https://www.fao.org/3/y1579e/y1579e03.htm (accessed on 23 January 2023).
- Rabinowitz, P.M.; Odofin, L.; Dein, F.J. From “us vs. them” to “shared risk”: Can animals help link environmental factors to human health? EcoHealth 2008, 5, 224–229. [Google Scholar] [CrossRef]
- Santos, J.; Ramos, C.; Vaz-Velho, M.; Vasconcelos Pinto, M. Occupational Exposure to Biological Agents. Adv. Saf. Manag. Hum. Perform. 2020, 1204, 61–67. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Domestic waste composting facilities: A review of human health risks. Environ. Int. 2009, 35, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Directive 89/391/EEC-OSH “Framework Directive”|Safety and Health at Work EU-OSHA. Available online: https://osha.europa.eu/pt/legislation/directives/the-osh-framework-directive/1 (accessed on 28 October 2022).
- Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the Protection of Workers from Risks Related to Exposure to Biological Agents at Work (Seventh Individual Directive within the Meaning of Article 16(1) of Directive 89/391/EEC). 2000. Volume 262. Available online: https://osha.europa.eu/en/legislation/directives/exposure-to-biological-agents/77 (accessed on 28 October 2022).
- OSHA Biological Agents and Prevention of Work-Related Diseases: A Review|Safety and Health at Work EU-OSHA. Available online: https://osha.europa.eu/en/publications/review-specific-work-related-diseases-due-biological-agents (accessed on 27 February 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269, W64. [Google Scholar] [CrossRef] [PubMed]
- Brauner, P.; Klug, K.; Jäckel, U. Eggshells as a source for occupational exposure to airborne bacteria in hatcheries. J. Occup. Environ. Hyg. 2016, 13, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Brauner, P.; Gromöller, S.; Pfeifer, Y.; Wilharm, G.; Jäckel, U. Hatchery workers’ IgG antibody profiles to airborne bacteria. Int. J. Hyg. Environ. Health 2017, 220, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Fallschissel, K.; Klug, K.; Kampfer, P.; Jäckel, U. Detection of Airborne Bacteria in a German Turkey House by Cultivation-Based and Molecular Methods. Ann. Occup. Hyg. 2010, 54, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Fallschissel, K.; Kampfer, P.; Jäckel, U. Direct Detection of Salmonella Cells in the Air of Livestock Stables by Real-Time PCR. Ann. Occup. Hyg. 2009, 53, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Gutarowska, B.; Szulc, J.; Nowak, A.; Otlewska, A.; Okrasa, M.; Jachowicz, A.; Majchrzycka, K. Dust at Various Workplaces—Microbiological and Toxicological Threats. Int. J. Environ. Res. Public Health 2018, 15, 877. [Google Scholar] [CrossRef]
- Martin, E.; Fallschissel, K.; Kämpfer, P.; Jäckel, U. Detection of Jeotgalicoccus spp. in poultry house air. Syst. Appl. Microbiol. 2010, 33, 188–192. [Google Scholar] [CrossRef]
- Martin, E.; Dziurowitz, N.; Jäckel, U.; Schäfer, J. Detection of Airborne Bacteria in a Duck Production Facility with Two Different Personal Air Sampling Devices for an Exposure Assessment. J. Occup. Environ. Hyg. 2015, 12, 77–86. [Google Scholar] [CrossRef]
- Martin, E.; Jäckel, U. Characterization of bacterial contaminants in the air of a duck hatchery by cultivation based and molecular methods. J. Environ. Monit. 2011, 13, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.; Bierbaum, G.; Kreyenschmidt, J.; Schmithausen, R.; Sib, E.; Schmoger, S.; Käsbohrer, A.; Hammerl, J. Clinically Relevant Escherichia coli Isolates from Process Waters and Wastewater of Poultry and Pig Slaughterhouses in Germany. Microorganisms 2021, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Bródka, K.; Kozajda, A.; Buczyńska, A.; Szadkowska-Stańczyk, I. The variability of bacterial aerosol in poultry houses depending on selected factors. Int. J. Occup. Med. Environ. Health 2012, 25, 281–293. [Google Scholar] [CrossRef]
- Lawniczek-Walczyk, A.; Gorny, R.L.; Golofit-Szymczak, M.; Niesler, A.; Wlazlo, A. Occupational exposure to airborne microorganisms, endotoxins and β-glucans in poultry houses at different stages of the production cycle. Ann. Agric. Environ. Med. 2013, 20, 259–268. [Google Scholar] [PubMed]
- Plewa, K.; Lonc, E. Seasonal biodiversity of pathogenic fungi in farming air area. Case Study Wiad Parazytol. 2011, 57, 118–122. [Google Scholar]
- Pomorska, D.; Larsson, L.; Skórska, A.; Sitkowska, J.; Dutkiewicz, J. Levels of Bacterial Endotoxin in the Samples of Settled Dust Collected in Animal Houses. Bull. Vet. Inst. Pulawy. 2009, 53, 27–41. [Google Scholar]
- Pomorska, D.; Larsson, L.; Skórska, C.; Sitkowska, J.; Dutkiewicz, J. Levels of Bacterial Endotoxin in Air of Animal Houses Determined with the Use of Gas Chromatography—Mass Spectrometry and Limulus Test. Ann. Agric. Environ. Med. 2007, 14, 291–298. [Google Scholar] [PubMed]
- Skóra, J.; Matusiak, K.; Wojewódzki, P.; Nowak, A.; Sulyok, M.; Ligocka, A.; Okrasa, M.; Hermann, J.; Gutarowska, B. Evaluation of Microbiological and Chemical Contaminants in Poultry Farms. Int. J. Environ. Res. Public Health 2016, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Sowiak, M.; Bródka, K.; Kozajda, A.; Buczyńska, A.; Szadkowska-Stańczyk, I. Fungal Aerosol in the Process of Poultry Breeding—Quantitative and Qualitative Analysis. Med. Pr. 2012, 63, 1–10. [Google Scholar]
- Amador, P.; Duarte, I.M.; Roberto da Costa, R.P.; Fernandes, R.; Prudêncio, C. Characterization of Antibiotic Resistance in Enterobacteriaceae From Agricultural Manure and Soil in Portugal. Soil Sci. 2017, 182, 292–301. [Google Scholar] [CrossRef]
- Viegas, C.; Carolino, E.; Malta-Vacas, J.; Sabino, R.; Viegas, S.; Veríssimo, C. Fungal Contamination of Poultry Litter: A Public Health Problem. J. Toxicol. Environ. Health Part A 2012, 75, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; dos Santos, M.; Carolino, E.; Sabino, R.; Quintal Gomes, A.; Viegas, S. Slaughterhouses Fungal Burden Assessment: A Contribution for the Pursuit of a Better Assessment Strategy. Int. J. Environ. Res. Public Health 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Veiga, L.; Figueredo, P.; Almeida, A.; Carolino, E.; Sabino, R.; Veríssimo, C.; Viegas, C. Occupational exposure to aflatoxin B1 : The case of poultry and swine production. World Mycotoxin J. 2013, 6, 309–315. [Google Scholar] [CrossRef]
- De Marco, M.A.; Delogu, M.; Facchini, M.; Di Trani, L.; Boni, A.; Cotti, C.; Graziosi, G.; Venturini, D.; Regazzi, D.; Ravaioli, V.; et al. Serologic Evidence of Occupational Exposure to Avian Influenza Viruses at the Wildfowl/Poultry/Human Interface. Microorganisms 2021, 9, 2153. [Google Scholar] [CrossRef]
- Paba, E.; Chiominto, A.; Marcelloni, A.M.; Proietto, A.R.; Sisto, R. Exposure to Airborne Culturable Microorganisms and Endotoxin in Two Italian Poultry Slaughterhouses. J. Occup. Environ. Hyg. 2014, 11, 469–478. [Google Scholar] [CrossRef]
- Patuzzi, I.; Orsini, M.; Cibin, V.; Petrin, S.; Mastrorilli, E.; Tiengo, A.; Gobbo, F.; Catania, S.; Barco, L.; Ricci, A.; et al. The Interplay between Campylobacter and the Caecal Microbial Community of Commercial Broiler Chickens over Time. Microorganisms 2021, 9, 221. [Google Scholar] [CrossRef]
- Schreuder, J.; Velkers, F.C.; Bouwstra, R.J.; Beerens, N.; Stegeman, J.A.; de Boer, W.F.; van Hooft, P.; Elbers, A.R.W.; Bossers, A.; Jurburg, S.D. An observational field study of the cloacal microbiota in adult laying hens with and without access to an outdoor range. Anim. Microbiome 2020, 2, 28. [Google Scholar] [CrossRef]
- Doménech, E.; Jiménez-Belenguer, A.; Pérez, R.; Ferrús, M.A.; Escriche, I. Risk characterization of antimicrobial resistance of Salmonella in meat products. Food Control 2015, 57, 18–23. [Google Scholar] [CrossRef]
- Haas, D.; Posch, J.; Schmidt, S.; Wüst, G.; Sixl, W.; Feierl, G.; Marth, E.; Reinthaler, F.F. A case study of airborne culturable microorganisms in a poultry slaughterhouse in Styria, Austria. Aerobiologia 2005, 21, 193–201. [Google Scholar] [CrossRef]
- Lugauskas, A.; Krikstaponis, A.; Sveistyte, L. Airborne fungi in industrial environments--potential agents of respiratory diseases. Ann. Agric. Environ. Med. 2004, 11, 19–25. [Google Scholar]
- Huneau-Salaün, A.; Le Bouquin, S.; Bex-Capelle, V.; Huonnic, D.; Balaine, L.; Guillam, M.-T.; Squizani, F.; Segala, C.; Michel, V. Endotoxin concentration in poultry houses for laying hens kept in cages or in alternative housing systems. Br. Poult. Sci. 2011, 52, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Heuer, O.E.; Pedersen, K.; Andersen, J.S.; Madsen, M. Vancomycin-Resistant Enterococci (VRE) in Broiler Flocks 5 Years after the Avoparcin Ban. Microb. Drug Resist. 2002, 8, 133–138. [Google Scholar] [CrossRef]
- Radon, K.; Weber, C.; Iversen, M.; Danuser, B.; Pedersen, S.; Nowak, D. Exposure assessment and lung function in pig and poultry farmers. Occup. Environ. Med. 2001, 58, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Luiken, R.E.C.; Van Gompel, L.; Bossers, A.; Munk, P.; Joosten, P.; Hansen, R.B.; Knudsen, B.E.; García-Cobos, S.; Dewulf, J.; Aarestrup, F.M.; et al. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ. Int. 2020, 143, 105971. [Google Scholar] [CrossRef] [PubMed]
- Radon, K.; Danuser, B.; Iversen, M.; Monso, E.; Weber, C.; Hartung, J.; Donham, K.J.; Palmgren, U.; Nowak, D. Air Contaminants in Different European Farming Environments. Ann. Agric. Environ. Med. 2002, 9, 41–48. [Google Scholar]
- Bai, H.; He, L.-Y.; Wu, D.-L.; Gao, F.-Z.; Zhang, M.; Zou, H.-Y.; Yao, M.-S.; Ying, G.-G. Spread of airborne antibiotic resistance from animal farms to the environment: Dispersal pattern and exposure risk. Environ. Int. 2022, 158, 106927. [Google Scholar] [CrossRef]
- Gao, M.; Jia, R.; Qiu, T.; Han, M.; Wang, X. Size-related bacterial diversity and tetracycline resistance gene abundance in the air of concentrated poultry feeding operations. Environ. Pollut. 2017, 220, 1342–1348. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Wang, Y.; Li, B.; Wang, Y. Spatiotemporal variations in the association between particulate matter and airborne bacteria based on the size-resolved respiratory tract deposition in concentrated layer feeding operations. Environ. Int. 2021, 150, 106413. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, N.; Liu, F.; Liu, W.J.; Bi, Y.; Zhang, Z.; Ma, S.; Cao, J.; Song, X.; Wang, A.; et al. More diversified antibiotic resistance genes in chickens and workers of the live poultry markets. Environ. Int. 2021, 153, 106534. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Xu, E.; Jiang, L.; Tang, J.; Li, M.; Zhao, X.; Chen, G.; Zhu, H.; Yu, X.; et al. Bacterial communities in PM2.5 and PM10 in broiler houses at different broiler growth stages in spring. Pol. J. Vet. Sci. 2019, 22, 495–504. [Google Scholar]
- Jo, W.-K.; Kang, J.-H. Exposure Levels of Airborne Bacteria and Fungi in Korean Swine and Poultry Sheds. Arch. Environ. Occup. Health 2005, 60, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Han, J.; Lee, Y.-K.; Kim, W.; Lee, S.-J. Bioaerosol exposure by farm type in Korea. Ann. Agric. Environ. Med. 2022, 29, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Kim, K.-Y. On-Site Investigation of Airborne Bacteria and Fungi According to Type of Poultry Houses in South Korea. Processes 2021, 9, 1534. [Google Scholar] [CrossRef]
- Roque, K.; Lim, G.-D.; Jo, J.-H.; Shin, K.-M.; Song, E.-S.; Gautam, R.; Kim, C.-Y.; Lee, K.; Shin, S.; Yoo, H.-S.; et al. Epizootiological characteristics of viable bacteria and fungi in indoor air from porcine, chicken, or bovine husbandry confinement buildings. J. Vet. Sci. 2016, 17, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Omeira, N.; Barbour, E.K.; Nehme, P.A.; Hamadeh, S.K.; Zurayk, R.; Bashour, I. Microbiological and chemical properties of litter from different chicken types and production systems. Sci. Total Environ. 2006, 367, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Moniri, R.; Dastehgoli, K. Fluoroquinolone-resistant Escherichia coli isolated from healthy broilers with previous exposure to fluoroquinolones: Is there a link? Microb. Ecol. Health Dis. 2005, 17, 69–74. [Google Scholar] [CrossRef]
- Hong, P.-Y.; Li, X.; Yang, X.; Shinkai, T.; Zhang, Y.; Wang, X.; Mackie, R.I. Monitoring airborne biotic contaminants in the indoor environment of pig and poultry confinement buildings. Environ. Microbiol. 2012, 14, 1420–1431. [Google Scholar] [CrossRef]
- Lee, S.-A.; Adhikari, A.; Grinshpun, S.A.; McKay, R.; Shukla, R.; Reponen, T. Personal Exposure to Airborne Dust and Microorganisms in Agricultural Environments. J. Occup. Environ. Hyg. 2006, 3, 118–130. [Google Scholar] [CrossRef]
- Nonnenmann, M.W.; Bextine, B.; Dowd, S.E.; Gilmore, K.; Levin, J.L. Culture-Independent Characterization of Bacteria and Fungi in a Poultry Bioaerosol Using Pyrosequencing: A New Approach. J. Occup. Environ. Hyg. 2010, 7, 693–699. [Google Scholar] [CrossRef]
- Peng, M.; Biswas, D. Environmental Influences of High-Density Agricultural Animal Operation on Human Forearm Skin Microflora. Microorganisms 2020, 8, 1481. [Google Scholar] [CrossRef]
- Schrader, J.S.; Singer, R.S.; Atwill, E.R. A Prospective Study of Management and Litter Variables Associated with Cellulitis in California Broiler Flocks. Avian Dis. 2004, 48, 522–530. [Google Scholar] [CrossRef]
- Stern, N.J.; Robach, M.C. Enumeration of Campylobacter spp. in Broiler Feces and in Corresponding Processed Carcasses. J. Food Prot. 2003, 66, 1557–1563. [Google Scholar] [CrossRef]
- Just, N.; Kirychuk, S.; Gilbert, Y.; Létourneau, V.; Veillette, M.; Singh, B.; Duchaine, C. Bacterial diversity characterization of bioaerosols from cage-housed and floor-housed poultry operations. Environ. Res. 2011, 111, 492–498. [Google Scholar] [CrossRef]
- Thibodeau, A.; Fravalo, P.; Laurent-Lewandowski, S.; Guévremont, E.; Quessy, S.; Letellier, A. Presence and characterization of Campylobacter jejuni in organically raised chickens in Quebec. Can. J. Vet. Res. 2011, 75, 298–307. [Google Scholar]
- Ahmed, M.F.E.; Ramadan, H.; Seinige, D.; Kehrenberg, C.; Abd El-Wahab, A.; Volkmann, N.; Kemper, N.; Schulz, J. Occurrence of extended-spectrum beta-lactamase-producing Enterobacteriaceae, microbial loads, and endotoxin levels in dust from laying hen houses in Egypt. BMC Vet. Res. 2020, 16, 301. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.H.A.; Elmorsy, T.H.; Tarwater, P.M.; Green, C.F.; Gibbs, S.G. Air biocontamination in a variety of agricultural industry environments in Egypt: A pilot study. Aerobiologia 2010, 26, 223–232. [Google Scholar] [CrossRef]
- Younis, F.; Salem, E.; Salem, E. Respiratory health disorders associated with occupational exposure to bioaerosols among workers in poultry breeding farms. Environ. Sci. Pollut. Res. Int. 2020, 27, 19869–19876. [Google Scholar] [CrossRef] [PubMed]
- Bertolatti, D.; O’Brien, F.; Grubb, W. Characterization of drug-resistant Staphylococcus aureus isolated from poultry processing plants in Western Australia. Int. J. Environ. Health Res. 2003, 13, 43–54. [Google Scholar] [CrossRef] [PubMed]
- van Gerwe, T.; Miflin, J.K.; Templeton, J.M.; Bouma, A.; Wagenaar, J.A.; Jacobs-Reitsma, W.F.; Stegeman, A.; Klinkenberg, D. Quantifying Transmission of Campylobacter jejuni in Commercial Broiler Flocks. Appl. Environ. Microbiol. 2009, 75, 625–628. [Google Scholar] [CrossRef]
- Banerjee, A.; Bardhan, R.; Chowdhury, M.; Joardar, S.N.; Isore, D.P.; Batabyal, K.; Dey, S.; Sar, T.K.; Bandyopadhyay, S.; Dutta, T.K.; et al. Characterization of beta-lactamase and biofilm producing Enterobacteriaceae isolated from organized and backyard farm ducks. Lett. Appl. Microbiol. 2019, 69, 110–115. [Google Scholar] [CrossRef]
- Chen, G.; Ma, D.; Huang, Q.; Tang, W.; Wei, M.; Li, Y.; Jiang, L.; Zhu, H.; Yu, X.; Zheng, W.; et al. Aerosol Concentrations and Fungal Communities Within Broiler Houses in Different Broiler Growth Stages in Summer. Front. Vet. Sci. 2021, 8, 775502. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications-detail-redirect/9789240060241 (accessed on 22 February 2023).
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 1–2. [Google Scholar] [CrossRef]
- UNEP Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance. Available online: http://www.unep.org/resources/superbugs/environmental-action (accessed on 27 February 2023).
- Viegas, C.; Cervantes, R.; Dias, M.; Gomes, B.; Pena, P.; Carolino, E.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S.; et al. Unveiling the Occupational Exposure to Microbial Contamination in Conservation-Restoration Settings. Microorganisms 2022, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Gomes, B.; Dias, M.; Carolino, E.; Aranha Caetano, L. Aspergillus Section Fumigati in Firefighter Headquarters. Microorganisms 2021, 9, 2112. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Dias, M.; Gomes, B.; Cervantes, R.; Pena, P.; Viegas, S.; Viegas, C. Microbial Occupational Exposure Assessments in Sawmills—A Review. Atmosphere 2022, 13, 266. [Google Scholar] [CrossRef]
- Gomes, B.; Pena, P.; Cervantes, R.; Dias, M.; Viegas, C. Microbial Contamination of Bedding Material: One Health in Poultry Production. Int. J. Environ. Res. Public Health 2022, 19, 16508. [Google Scholar] [CrossRef]
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. Worlds Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Articles published in the English language | Articles published in other languages |
| Articles published from 1 January 2000 to 20 January 2023 | Articles published prior to 2000 |
| Articles reporting findings from any country | Articles related to biocontrol efficacy or related to clinical trials |
| Articles related to microbial exposure from poultries and related products | Articles related to biocontrol efficacy or without mention of microbial exposure or metabolites |
| Original scientific articles on the topic | Abstracts of congresses, reports, reviews/state of the art articles |
| Database | Title | Country | Environment Assessed | Objective (Occupational/Food Safety/Public Health/Animal Health) | Microorganisms and Metabolites | Analyzed Matrices | Sampling Methods | Analytical Methods | Main Findings | References |
|---|---|---|---|---|---|---|---|---|---|---|
| PubMed | Occupational exposure to aflatoxin (AFB₁) in poultry production | Portugal | Poultry farm | Occupational health | Fungi Mycotoxins | Air samples Surface swabs Litter collection (poultry) Floor coverage collection (swine) Workers’ biological samples (blood: poultry farms n = 31) | Active methods Impaction flow rate = 140 L/min) Passive methods (material collection; swabs) | Culture-based methods ELISA | Eighteen poultry workers (58.6%) and six workers from the swine production facilities (54.5%) showed detectable levels of aflatoxin B1 (AFB1). The findings indicate that AFB1 inhalation exposure occurs in both occupational settings, posing an additional risk that must be identified, assessed, and avoided. | [40] |
| Bioaerosol exposure by farm type in Korea | Korea | Animal farms (open field, greenhouse and livestock facilities: poultry, swine, and cattle) | Occupational health | Bacteria Fungi Endotoxins | Air samples (open field farms: personal n = 4, environment n = 20) Greenhouses: personal n = 32, environment n = 159 Livestock facilities: environment n = 21, poultry n = 9; swine n = 5; cattle n = 5) | Active methods (single-stage impactor, flow rate = 28.3 L/min; button aerosol sampler with sterilized gelatin filters, flow rate = 4 L/min; two-stage cassette with a glass fiber filter for endotoxins, flow rate = 2 L/min) | Culture-based methods Limulus amoebocyte lysate (LAL) assay (endotoxins) | The highest endotoxin concentration was at hog farms (160.35 EU/m3), followed by poultry houses (103 EU/m3) and cowsheds (28 EU/m3). The measured levels of endotoxins at hog farms and poultry houses exceeded exposure limits. The concentrations of personal samples were higher than those of the area samples. Exposure levels in residential and rest areas were significantly higher than in the control areas, possibly being contaminated from bioaerosols generated in the workplace. | [59] | |
| Serologic Evidence of Occupational Exposure to Avian Influenza Viruses at the Wildfowl/Poultry/Human Interface | Italy | Poultry farm (n = 17) | Occupational health | Viruses (avian influenza viruses) | Bird cloacal swabs (n = 2542) Oropharyngeal swabs (n = 1045) Avian sera (n = 2688) Human sera (n = 57 workers) and blood samples | Passive methods (swabs, material collection) Biological samples | Virological and serological assays(hemagglutination inhibition assay; enzyme-linked immunosorbent assay (ELISA)) | Antibodies specific to avian influenza viruses (AIH)-H3, AIV-H6, AIV-H8, and AIV-H9 were found in three poultry workers (PWs). The data obtained emphasize the occupational risk posed by zoonotic AIV strains. These findings highlight the crucial role of integrated occupational medicine and veterinary avian influenza virus surveillance aimed to further assess the health risk at the wildfowl/poultry/human interface | [41] | |
| Spatiotemporal variations in the association between particulate matter and airborne bacteria based on the size-resolved respiratory tract deposition in concentrated layer feeding operations | China | Poultry farms (n = 9) | Occupational health | Bacteria | Air samples (n = 8) | Active methods (Andersen eight-stage samplers, n = 2; Andersen six-stage samplers, n = 2, flow rate = 1cubic foot/min) Particulate matter (PM) collected on the surface of a glass fiber filter membrane with a diameter of 81 mm and pore size of 2.0 μm) | Culture-based methods | The emissions of PM and airborne bacteria (AB) from the poultry houses resulted in high PM and AB concentrations in the surrounding areas. Particles with diameters ranging from 2.1–4.7 μm carried the most airborne bacteria. Therefore, particles with those dimensions should be the focus of future experimental research on occupational exposure, air quality improvement, and airborne transmission. | [55] | |
| Clinically Relevant Escherichia coli Isolates from Process Waters and Wastewater of Poultry and Pig Slaughterhouses in Germany | Germany | Poultry (n = 2) and pig (n = 2) slaughterhouses | Environmental health and food safety | Bacteria | Water samples from poultry (n = 82) and pigs (n = 67) | Passive methods (material collection) | Culture-based methods Antimicrobial susceptibility Molecular tools (whole-genome sequencing) | Selected E. coli isolates (n = 71) constituted a reservoir for 53 different antimicrobial resistance determinants and were assigned various sequence types, including high-risk clones involved in human infections worldwide. Through cross-contamination, these multidrug-resistant E. coli pathotypes may be introduced into the food chain. Moreover, inadequate wastewater treatment may contribute to bacterial dissemination into surface waters. | [29] | |
| The Interplay between Campylobacter and the Caecal Microbial Community of Commercial Broiler Chickens over Time | Italy | Poultry farms (n = 4) | Food safety | Bacteria | Cecal swabs (n = 320) | Passive methods (swabs) | Culture-based methods Molecular tools (RT-PCR/amplicon PCR) | Two out of four farms showed Campylobacter infection at different time points. Moreover, Campylobacter colonization dramatically influenced the microbiota richness, although to a different extent depending on the infection timing. Briefly, the evidence obtained in this study can be used to identify options to minimize the incidence of infection in primary production based on the targeted influence of birds’ intestinal microbiota, in order to reduce the risk of human exposure to Campylobacter by chicken meat consumption. | [43] | |
| Environmental Influences of High-Density Agricultural Animal Operation on Human Forearm Skin Microflora | USA | Animal farms (dairy and integrated farms: cattle, chicken, pig, sheep; n = 20) | Occupational health | Bacteria | Skin swabs from farm workers (n = 20) | Passive methods (swabs) | Molecular tools (16s rRNA gene sequencing) | Different microbial compositional patterns were found on skin of workers of different animal commodities. The alterations of forearm skin microflora in farm workers, influenced by their frequent farm animal operations, may increase their risk of skin infections with unusual pathogens and epidermal diseases. | [67] | |
| Occurrence of extended-spectrum betalactamase- producing Enterobacteriaceae, microbial loads, and endotoxin levels in dust from laying hen houses in Egypt | Egypt | Poultry farms (n = 28) | Occupational health and food safety | Bacteria Fungi Endotoxins | Settled dust from elevated surfaces inside the barn (n = 10), including the drinking system line, feeding system line, and ventilation opening | Passive methods (dust collection) | Culture-based methods Antimicrobial susceptibility MALDI-TOF (bacterial identification) LAL | Dust in Egyptian laying hen houses contains high concentrations of microorganisms and endotoxins, which might impair the health of birds and farmers when inhaled. Furthermore, laying hens in Egypt seem to be a reservoir for beta-lactamase (ESBL)-producing Enterobacteriaceae. Overall, farmers are at risk of exposure to ESBL-producing bacteria, and colonized hens might transmit these bacteria to the food chain. | [72] | |
| An observational field study of the cloacal microbiota in adult laying hens with and without access to an outdoor range | Netherlands | Poultry farms (n = 8) | Animal health | Bacteria | Cecal swabs (n = 100) | Passive methods (swabs) | Culture-based methods Molecular tools (16s rRNA gene sequencing) | Bacterial diversity was higher in Indoor layers than in outdoor layers, and indoor layers also had more variation in their bacterial community composition. No phyla or genera were found to be differentially abundant between indoor and outdoor poultry houses. The poultry house, farm, and rearing flock play a much greater role in determining the cloacal microbiota composition of adult laying hens. | [44] | |
| Dust at Various Workplaces—Microbiological and Toxicological Threats | Poland | Several workplaces (n = 4)(cement plant, composting plant, poultry farm, and cultivated area) | Occupational health | Bacteria Fungi | Air (n = 1) and settled dust (n = 1) | Active method (Air: DustTrak™ DRX Aerosol Monitor 8533 portable laser photometer, TSI) Passive methods (dust collection) | Culture-based methods Molecular tools (PCR) Cytotoxicity assay (A-549 MTT test) | Settled dust samples evidence the presence of 139 bacterial genera belonging to 8 classes and 107 fungal genera from 21 classes. In all tested settled dust samples, potentially allergenic molds were present, including Aspergillus sp. and Penicillium sp. (cement and composting plants) and Cladosporium sp. (cement plants and poultry farms) | [25] | |
| Hatchery workers’ IgG antibody profiles to airborne bacteriaPaul | Germany | Animal farms (duck hatchery) (n = 11) | Occupational health | Bacteria | Air samples Human sera (n = 10 workers | Active methods (filtration device using gelatin filters and polycarbonate filter, flow rate = 1.8 m3/h) Biological samples | Molecular tools (pulsed-field gel electrophoresis (PFGE); multiplex PCR and blaOXA-51-like and 16s rRNA gene sequencing) Fluorescence quantification | Despite long-term bioaerosol exposure, hatchery workers’ IgG antibody profiles to tested antigens did not differ substantially from those of the control group. However, increased workers’ titers to Acinetobacter baumannii and clinical relevance of this species should lead to further investigations regarding potential involvement in pathogenesis of occupational respiratory disorders. | [22] | |
| Epizootiological characteristics of viable bacteria and fungi in indoor air from porcine, chicken, or bovine husbandry confinement buildings | Korea | Animal farms (Swine, n = 5; chicken, n = 12; and cattle farms, n = 5) | Occupational and animal health | Bacteria Fungi Endotoxins | Air samples | Active methods (cascade impactor, flow rate = 28.3 ç/min, 20 min; PVC membrane filters (SKC) with 37 mm cassettes, flow rate = 2.0 L/min for 8 h, endotoxins) | Culture-based methods LAL | In chicken farms, a total of 22 Gram-positive bacterial species, three Gram-negative bacterial species, and five fungal species were identified. All broiler farms exceeded the recommended stocking density (0.066 m2/head), which may have led to the higher endotoxin concentrations in indoor dust from chicken farms than pig or cattle farms. Monitoring the indoor airborne endotoxin level was also found to be critical for risk assessment of health for animals or workers. | [61] | |
| Eggshells as a source for occupational exposure to airborne bacteria in hatcheries | Germany | Animal farms (turkey hatchery) | Occupational health | Bacteria | Air samples Turkey eggshell (n = 4) | Active methods (filtration devices (MD8 aluminum stacks, Sartorius, Göttingen, Germany) Bioaerosols were collected on gelatin filters (Ø 78 mm, 3.0 μm pore size (flow rate = 1.8 m3/h) Passive methods (material collection) | Culture-based methods Fluorescence quantification Molecular tools (16s rRNA gene sequencing) | Enterococcus gallinarum was found as the predominant species on turkey eggshells, both have been classified as risk group 2 microorganisms. During different work activities with poult and eggshell handling, concentrations of airborne Enterococci up to 1.3×104 cfu/m3 were found. After hatching of turkey poults, hatcher incubators and eggshell fragments provide appropriate conditions for excessive bacterial growth. Thus, high bacterial loads on eggshell fragments are a source of potentially harmful bioaerosols caused by air flows, poult activity, and handling of equipment. | [21] | |
| Evaluation of Microbiological and Chemical Contaminants in Poultry Farms | Poland | Poultry farms (n = 13) | Occupational and animal health | Bacteria | Air samples (n = 5) Settled dust (n = 3) | Active methods (aspirator (EAS 1203; Emio, Wrocław, Poland) DustTrak™ DRX aerosol monitor) Passive methods (dust collection) | Culture-based methods Cytotoxicity assay (A-549 MTT test) Chemical assessment (gas chromatographic and spectrophotometric methods (LC-MS/MS: secondary metabolites; GC/MS) | The airborne total dust concentration at poultry farms averaged 1.44 mg/m3 with a high percentage of the PM10 fraction. Microorganism concentrations in the settled dust were: 3.2 × 109 cfu/g for bacteria and 1.2 × 106 cfu/g for fungi. Potential pathogens (Enterococcus spp., Escherichia coli, Salmonella spp., Aspergillus fumigatus, Paecilomyces variotii) were also found. In conclusion, settled dust can be a carrier of microorganisms, odors, and secondary metabolites in poultry farms, which can be harmful to workers’ health. | [35] | |
| Detection of Airborne Bacteria in a Duck Production Facility with Two Different Personal Air Sampling Devices for an Exposure Assessment | Germany | Poultry farm (n = 2) | Occupational health | Bacteria | Air samples (n = 6) | Active methods (PGP filtration device with polycarbonate filters, pore size: 0.8 μm, Ø 37 mm; two-stage bioaerosol Cyclone 251, flow rate = 3.5 L/min) | Fluorescence quantification Molecular tools (restriction fragment length polymorphism (RFLP) analysis; 16s rRNA gene sequencing) | Detailed 16S rRNA gene sequence analyses showed potential exposure to risk group 2 bacteria at the hatchery workplace. A size fractionated sampling device revealed that pathogenic bacteria would reach the inhalable, thorax, and possibly alveolar fraction of lungs. | [27] | |
| Scopus | Detection of Airborne Bacteria in a German Turkey House by Cultivation-Based and Molecular Methods | Germany | Poultry farms (n = 2) | Occupational health | Bacteria | Air samples (n = 2) | Active methods (filtration devices (MD8 aluminum stacks; Sartorius, Göttingen, Germany, through polycarbonate membrane filters, 0.80 lm pore size, flow rate = 28.1 L/min; all-glass impingers, AGI- 30; Ace Glass Inc., flow rate = 11.41 L/min) | Culture-based methods Molecular tools (PCR; 16s rRNA gene sequencing) | Microbial species with a potential health risk for employees (Acinetobacter johnsonii, Aerococcus viridans, Pantoea agglomerans, and Shigella flexneri) were identified. The animals seem to be the most important source of airborne microorganisms in the investigated turkey houses. | [23] |
| Scopus | Characterization of bacterial contaminants in the air of a duck hatchery by cultivation based and molecular methods | Germany | Poultry farm | Occupational health | Bacteria | Air samples (n = 10) | Active methods (filtration devices using polycarbonate filters (Ø 76 mm, 0.8 mm pore size) and gelatin filters (Ø 78 mm, 3.0 mm pore size, flow rate = 1.8 L/min)), MD8 aluminum stacks; Sartorius, Germany. | Culture-based methods Molecular tools (16s rRNA gene sequencing) | More than 50% of bacterial isolates were phylogenetically most closely related to bacterial species of risk group 2. There were high concentrations of risk group 2 bacteria, which have been implicated in different human respiratory disorders. Adequate breathing protection for employees is recommended during sorting of ducklings. | [28] |
| Monitoring airborne biotic contaminants in the indoor environment of pig and poultry confinement buildings | USA | Animal farms (poultry and swine farm) | Occupational health | Bacteria | Air samples (n = 48) | Active methods (isokinetic sampling nozzle assembled with a polycarbonate cassette that housed a sterilized 37 mm glass fiber filter) | Molecular tools (16s rRNA pyrose sequencing; tetracycline resistance genes) | Bioaerosols in the confinement buildings were sporadically associated with genera of potential pathogens. Bacterial lineages present in the poultry bioaerosols clustered apart from those present in the pig bioaerosols. The abundance of different classes of tetracycline resistance genes also differed among the different animal confinement buildings. | [64] | |
| On-Site Investigation of Airborne Bacteria and Fungi According to Type of Poultry Houses in South Korea | Korea | Poultry farms (caged layer house n = 9; broiler house n = 9; layer house with manure belt n = 9) | Occupational health | Bacteria Fungi | Air samples (n = 5) | Active methods (one-stage viable particulate cascade impactor, Model 10–800, Andersen Inc., Bayport, MN, USA, flow rate of 28.3 L/min) | Culture-based methods | Among poultry buildings, the broiler house showed the highest exposure level and emission rate of total airborne bacteria and fungi, followed by the layer house with manure belt and the caged layer house. The highest exposure level and emission rate of airborne microorganisms found in the broiler house could be attributed to sawdust, which can be dispersed into the air by the movement of the poultry when it is utilized as bedding material. | [60] | |
| Exposure to Airborne Culturable Microorganisms and Endotoxin in Two Italian Poultry Slaughterhouses | Italy | Poultry slaughterhouses (n = 2) | Occupational health | Bacteria Fungi Endotoxins | Air samples (Poultry A n = 273; Poultry B n = 210) Workers’ personal air samples (Poultry A n = 5; Poultry B n = 2) | Active methods Portable air microbiological sampler -SAS Super ISO, PBI International, Milan, Italy, flow rate=100 L/min; Personal sampling pumps - Model Number SKC 224-PCXR8, Eighty Four, Pa., equipped with Button Aerosol Sampler and gelatin filters -GEL SKC, Inc., Pa, flow rate= 4 L/min; | Culture-based methods LAL | The microbial flora was dominated by Gram-negative and coagulase-negative staphylococci for bacteria and by species belonging to Cladosporium, Penicillium, and Aspergillus genera for molds. Overall, microbial levels were below the occupational limits. However, the microorganisms identified may exert adverse effects on exposed workers, in particular for those engaged in the early slaughtering stages, as evidenced by the presence of pathogenic species. Additionally, the detection of pathogenic bacteria near air handling units may constitute a risk to public health and of environmental pollution. | [42] | |
| Spread of airborne antibiotic resistance from animal farms to the environment: Dispersal pattern and exposure risk | China | Animal farms (poultry and dairy farm) | Human health due to environmental impact | Bacteria | Air samples (n = 4) Dust and animal feces samples | Active methods (portable high-volume sampler, HighBioTrap, Beijing dBlue Tech, Inc., Beijing, China, flow rate = 1000 L/min) Passive methods (material collection) | Culture-based methods Antimicrobial susceptibility Molecular tools (ABI QuantStudio™ 7 Flex RT-PCR; 16s rRNA gene sequencing) | Antibiotic resistance genes (ARGs) from bacteria were detected from upwind (50 m/100 m) and downwind (50 m/100 m/150 m) air environments, wherein at least 30% of bacterial taxa dispersed from the animal houses. Clinically important pathogens were identified in airborne culturable bacteria. Staphylococcus, Sphingomonas, and Acinetobacter genera were potential bacterial hosts of airborne ARGs. Airborne Staphylococcus spp. were isolated from the environment of the chicken farm (n = 148) and dairy farm (n = 87). | [53] | |
| Levels of bacterial endotoxin in air of animal houses determined with the use of gas chromatography—mass spectrometry and Limulus test | Poland | Animal farms (cow barns n = 4; piggeries n = 4; sheep sheds n = 4; poultry houses n = 4; horse stables n = 6) Buildings for storage of hay (n = 3) | Human health due to environmental impact and animal health | Endotoxins | Air samples (n = 2) | Active methods (portable single-unit aspirator AP-2A (TWO-MET, Zgierz, Poland) on pre-weighed glass fiber filters of diameter 37 mm and pore size 1.0 μm, flow rate = 2 L/min) | LAL Spectrophotometric methods (gas chromatography–tandem mass spectrometry (GC-MSMS) | The concentrations of airborne endotoxin determined with LAL test and GC-MSMS analysis exceeded the limits in most of the animal houses examined. Endotoxin in the concentrations detected in this study may present a respiratory hazard to both humans and livestock animals. | [34] | |
| Microbiological and chemical properties of litter from different chicken types and production systems | Lebanon | Poultry farms (n = 12) | Human health due to environmental impact and environmental health | Bacteria | Litter samples (n = 24) | Passive methods (material collection) | Culture-based methods Chemical analysis | Staphylococcus species were observed in the litter from free-range layers (p = 0.0077). Staphylococcus species in the litter as well as cadmium concentrations seem to be the most critical parameters presenting risks to the environment and human health. | [62] | |
| Quantifying Transmission of Campylobacter jejuni in Commercial Broiler Flocks | Australia | Poultry farms (n = 42) | Animal health | Bacteria | Surface swabs (fecal or cecal droppings, n = 10) | Passive methods (material collection) | Culture-based methods | The transmission rate estimate was 2.37 − 0.295 infections per infectious bird per day. Based on these results, colonized flocks consisting of 20,000 broilers would have an increase in within-flock prevalence to 95% within 4.4 to 7.2 days after colonization of the first broiler. Thus, interventions aimed at prevention of introduction and subsequent colonization by Campylobacter might be better targeted at the second half of the rearing period, which is considered a high-risk period. | [76] | |
| Presence and characterization of Campylobacter jejuni in organically raised chickens in Quebec | Canada | Poultry farms (n = 6) | Food safety | Bacteria | Cecal swabs (n = 30) Fecal matter (n = 30 g) Animal carcasses (birds, n = 10) | Passive methods (swabs, material collection) | Culture-based methods Antimicrobial susceptibility Molecular tools (PCR) | Campylobacter jejuni isolates were resistant to tetracycline, erythromycin, azithromycin, and clindamycin. Some organic chicken lots sampled in Quebec were positive for C. jejuni, which establishes this presence for the first time and suggests a possible contribution of these types of production to human campylobacteriosis. | [71] | |
| Risk characterization of antimicrobial resistance of Salmonella in meat products | Spain | Animal farms (poultry, pork, and beef farms; 95% industry and 5% retail) | Food safety | Bacteria | Animal carcasses (fresh poultry, n = 234); pork, n = 196); beef, n = 29; minced poultry, n = 151; pork, n = 1270; and beef, n = 170) | Passive methods (material collection) | Culture-based methods Antimicrobial susceptibility | Salmonella isolates found in poultry had a high level of resistance to nalidixic acid, while those found in pork were more resistant to tetracycline and ampicillin. Furthermore, 41% of Salmonella isolates were resistant to three or more antibiotics. Additionally, risk characterization was estimated. As a result, three cases were classified as “very high additional risk,” all of them in minced meat, two cases in poultry (gentamicin and nalidixic acid), and one in pork (ampicillin). | [45] | |
| Characterization of Antibiotic Resistance in Enterobacteriaceae From Agricultural Manure and Soil in Portugal | Portugal | Animal farms (poultry, n = 6) and dairy farms, n = 6) | Environmental health | Bacteria | Manure samples (n = 18) Soil samples (n = up to 15 cm) | Passive methods (material collection) | Culture-based methods Antimicrobial susceptibility Molecular tools (ARGs) | High multidrug resistance rates (>70%) were observed in both manure and soil samples. This resistance was higher in the poultry samples. Manured-soil isolates were more resistant to cefoxitin (91.7%), ulfamethoxazole/trimethoprim (79.2%), chloramphenicol (79.2%), and, to a lesser extent, tetracycline (12.5%). In short, the results obtained are important for soil management regarding resistance determinants spread through agricultural practices. | [37] | |
| Levels of bacterial endotoxin in the samples of settled dust collected in animal houses | Poland | Animal farms(poultry n = 4; sheep sheds n = 4; horse stables n = 6) | Occupational and animal health | Endotoxins | Settled dust samples (n = 14) | Passive methods (material collection) | LAL (endotoxins) Spectrophotometric methods (GC-MSMS) | The median concentrations of the endotoxin in dust determined with LAL tests in sheep sheds, poultry houses, and horse stables were 15,687.5 μg/g, 8081.8 μg/g, and 79.3 μg/g, respectively, while those determined with the GC-MSMS technique were 868.0 μg/g, 580.0 μg/g, and 496.0 μg/g, respectively. In conclusion, endotoxin in the concentrations detected in this study may present a respiratory hazard to both livestock animals and farm workers. | [33] | |
| Characterization of beta-lactamase and biofilm producing Enterobacteriaceae isolated from organized and backyard farm ducks | India | Animal farms (farm ducks, n = 8) | Human health due to environmental impact | Bacteria | Cloacal swabs (n = 202) | Passive methods (swabs) | Culture-based methods Antimicrobial susceptibility Molecular tools (PCR) | From 202 cloacal swabs of apparently healthy ducks, 109 (53–96%), 13 (6–44%), and 30 (14–85%) isolates were confirmed as E. coli, Salmonella, and Klebsiella pneumoniae, respectively. Most of the beta-lactamase and biofilm-producing Enterobactriaceae isolates exhibited phenotypical resistance against ampicillin, ampicillin/cloxacillin, and ceftriaxone. This study evidenced the ducks as a reservoir of beta-lactamase and biofilm-producing Enterobactriaceae which might enter the food chain to cause major public health hazards. | [77] | |
| More diversified antibiotic resistance genes in chickens and workers of the live poultry markets | China | Poultry farms (n = 21) and live poultry markets (LPMs) (n = 22) | Human health due to environmental impact | Bacteria | Bird fecal samples (n = 1215) Human fecal samples (n = 36) Material collection in 4 LPM environmental samples (soils, sediment, wastewater, and chopping boards, n = 4). | Passive methods (material collection) | Molecular tools (metagenomic sequencing (ARGs)) | Some mobile ARGs, such as mcr-1 and tet(X3), identified in chicken farm LPMs, LPM workers, and LPM environments, were also harbored by human clinical samples. Resistomes were significantly different between the LPM workers and those who have no contact with the LPMs, and more diversified ARGs (188 types) were observed in the LPM workers. It is also worth noting that mcr-10 was identified in both human (5.2%, 96/1859) and chicken (1.5%, 14/910) gut microbiomes. These findings highlight the live poultry trade as an ARG disseminator into LPMs. | [56] | |
| Bacterial diversity characterization of bioaerosols from cage-housed and floor-housed poultry operations | Canada | Poultry farms (n = 30) | Occupational health | Bacteria and endotoxins | Air samples from cage-housed (CH, n = 15) and floor-housed (FH, n = 15) poultry operations | Active methods (Marple cascade impactor with weighed radial slit polyvinyl chloride (PVC) filters connected to a constant air flow pump—Universal 224-PCXR4; SKC, Eighty Four, PA, USA, six stages were included, flow rate = 2 L/min, over 4 hs) | Molecular tools (PCR; denaturing gradient gel electrophoresis (DGGE)) LAL Spectrophotometric methods (GC-MSMS) | Dust, endotoxin, and bacteria were significantly higher in personal bioaerosols of FH poultry operations than CH bioaerosols. Personal CH bioaerosols have a greater prevalence of bacteria, some of which have been shown to cause respiratory dysfunction. Therefore, bacterial diversity may help to explain the greater prevalence of respiratory symptoms in workers from CH operations. | [70] | |
| Characterization of drug-resistant Staphylococcus aureus isolated from poultry processing plants in Western Australia | Australia | Poultry processing plants (n = 2) | Food safety | Bacteria | Samples from broiler chickens and turkeys (n = 104) during the processing Samples from defeathering machinery and bleed drains (n = 22) | UK | Culture-based methods Antimicrobial susceptibility Molecular tools | One hundred and twenty-six Staphylococcus aureus were isolated from two poultry processing plants in Western Australia. Antimicrobial-resistant S. aureus were recovered from live incoming birds, equipment, and processed carcasses in the two processing plants. Indeed, forty-six (36.5%) of the isolates were resistant to six or more of the antimicrobial agents tested. | [75] | |
| Vancomycin-Resistant Enterococci (VRE) in Broiler Flocks 5 Years after the Avoparcin Ban | Denmark | Poultry farms where avoparcin had previously been used (n = 31) Poultry farms without avoparcin (n = 12) | Food safety | Bacteria | Cloacal swabs (n = 10) | Passive methods (swabs) | Culture-based methods Antimicrobial susceptibility Molecular tools (vanA PCR) | VRE were isolated from 104 of 140 (74.3%) broiler flocks reared in broiler houses previously exposed to avoparcin on conventional and extensive indoor broiler farms. Results demonstrated the extensive occurrence of VRE in broiler flocks even 5 years after the avoparcin ban in Denmark. The extensive occurrence of VRE in broiler flocks reported in this study indicates that consumers may still be exposed to VRE from poultry products despite the avoparcin ban. | [49] | |
| Personal Exposure to Airborne Dust and Microorganisms in Agricultural Environments | USA | Animal farms (swine, poultry, and dairy, n = 3) and grain farms (n = 3) | Occupational health | Bacteria Fungi | Air samples (swine n = 5; poultry n = 2; dairy n = 5; corn harvesting n = 6; soybean n = 3) | Active methods (prototype personal sampling, consists of seven components in each of the two sampling lines: sampling probe Tygon tubing, adaptor, metal sampling chamber, optical particle counter, 25 mm filter cassette and pump, flow rate = 10 L/min) | Culture-based methods Antimicrobial susceptibility Molecular tools (vanA PCR) | A large fraction (up to 37%) of particles from 2–10 μm was found to be fungal spores. Each type of agricultural environment was found to have specific characteristics of exposure. Harvesting was dominated by exposure to large dust particles with a large fraction of fungal spores, whereas the particle size distributions in animal confinements were dominated by small particles. | [65] | |
| Farm dust resistomes and bacterial microbiomes in European poultry and pig farms | European countries (Belgium, Bulgaria, Denmark, France, Germany, Italy, the Netherlands, Poland, and Spain) | Animal farms (poultry n = 12; swine farms n = 19) | Occupational health | Bacteria | Dust collection by electrostatic dust collector (n = 3) Fecal samples from poultry (n = 35) and workers (n = 44) | Passive methods (material collection) | Molecular tools (metagenomic sequencing (ARGs)) | The farm dust resistome contained a large variety of ARGs; more than the animal fecal resistome. The farm dust resistome from European poultry and pig farms is equally or more abundant and rich than the resistome of poultry and pig feces and farmers. A positive association between on-farm antimicrobial usage in animals on the farm and the total abundance of the dust resistome was found. Briefly, poultry and pig farm dust resistomes are rich and abundant and associated with the fecal resistome of the animals and the dust bacterial microbiome | [51] | |
| Fluoroquinolone-resistant Escherichia coli isolated from healthy broiler s with previous exposure to fluoroquinolones: Is there a link? | Iran | Poultry farms (n = 7) | Human health due to environmental impact | Bacteria | Samples from broiler chickens and turkeys previously exposed to both quinolone (flumequine) and fluoroquinolone (n = 95) | UK | Culture-based methods Antimicrobial susceptibility | The differences between ciprofloxacin resistance rates in strains from chickens with previous exposure to fluoroquinolones compared with isolates from chickens without a history of drug use were significant (49.5% vs. 33.7%, p =/0.0461). It seems that use of fluoroquinolones constitutes a major selective pressure for resistance. | [63] | |
| Enumeration of Campylobacter spp. in Broiler Feces and in Corresponding Processed Carcasses | EUA | Poultry farms (n = 20) | Food safety | Bacteria | Bird fecal samples (n = 50) Bird carcasses before they entered the chill tank (n = 50) and after being fully processed (n = 50) | Passive methods (material collection) | Culture-based methods Antimicrobial susceptibility | Individual birds within each of the flocks involved in the current study were 70 to 100% colonized prior to loading and transport. Levels of Campylobacter spp. found in production and in processing were not strongly correlative, indicating the existence of complex parameters involving production factors and variables associated with flock transport and the processing of the broilers. The sources of Campylobacter sp. appear to be diverse, and discussion regarding the optimum approach for the control of the organism during poultry production remains lively. | [69] | |
| A prospective Study of Management and Litter Variables Associated with Cellulitis in California Broiler Flocks | USA | Poultry farms (n = 5) | Animal health | Bacteria | Litter samples (n = 3, 60 g) | Passive methods (material collection) | Culture-based methods | There was a positive association between the quantity of Gram-negative bacteria in the litter in the front third of the house (the brooding area) during the brooding period and the percentage of cellulitis. | [68] | |
| Fungal aerosol in the process of poultry breeding quantitative and qualitative analysis | Poland | Poultry farms (n = 5) | Occupational health | Fungi | Air samples (n = 11) | Active methods (filtration method, GilAir 5 pump—Sensidyne, Clearwater, Florida, USA; open-faced aerosol sampler Two-Met, Zgierz, Poland, with a GF/A filter, Whatman International Ltd., Maidstone, Kent, UK, of a 37 mm diameter, flow rate = 2 L/min) | Culture-based methods | In 45% of the taken samples, airborne mesophilic fungal levels exceeded the reference value recommended in Poland for occupational environment exposure. Briefly, facilities of poultry farms are contaminated with high concentrations of fungal aerosols, especially in the colder season. Additionally, potential pathogenic microorganisms were present, which may pose a risk to farm workers’ health. | [36] | |
| Seasonal biodiversity of pathogenic fungi in farming air area. Case study. | Poland | Poultry farm | Human health due to environmental impact | Fungi | Air samples (indoor n = 4, outdoor n = 4) | Active methods (impaction method, Merck MAS-100, flow rate = 100 L/min) | Culture-based methods | The most common airborne fungi, inside the poultry house, as well as in the surrounding areas, were Penicilium sp., Aspergillus sp., Cladosporium sp., and Alternaria sp. The majority of the identified fungal species were characterized as potential allergens and exposure to their spores may provoke immune response in susceptible individuals. | [32]94b | |
| Web of Science | Aerosol Concentrations and Fungal Communities Within Broiler Houses in Different Broiler Growth Stages in Summer | China | Poultry farms (n = 3) | Human health due to environmental impact and animal health | Fungi | Air samples (n = 3) | Active methods (Andersen six-stage sampler ZR-2001, Zhongrui, Qingdao, China, flow rate = 28.3 L/min, 2 min; biosampler (ZR- 2000, Zhongrui, Qingdao, China, flow rate = 5–35 L/min)) | Molecular tools (PCR) | The concentration of fungal aerosols in the poultry houses increased as the ages of the broilers increased, which was also accompanied by gradual increases in the variety and diversity indices of the fungal communities in the air of the poultry houses. Overall, the dominant fungal genera found may be harmful to the health of poultry and human beings. Thus, permanent monitoring of microbial air quality in chicken houses is necessary. | [78] |
| Respiratory health disorders associated with occupational exposure to bioaerosols among workers in poultry breeding farms | Egypt | Poultry farms (n = 10) | Occupational health | Bacteria Fungi | Air samples (n = 10) Swabs (workers’ nose and throat, n = 56) | Active methods (Andersen six-stage impactor, Model 10–710, Andersen Instruments, Atlanta, GA, USA, flow rate = 28.3 L/min, 0.5 to 2 min; spirometer, MEE Spiro PFT touch, Germany) Passive methods (swabs) | Culture-based methods Questionnaire Spirometric measures | The percentage of total positive cultured (bacterial and fungal) was 35.7% among the poultry breeding farm workers. About one third of the studied farm workers (30.4%) were a carrier for S. aureus in the nose and throat compared with 12.5% of the control group. Additionally, Aspergillus species were present in air samples as well as in human samples. These results suggest that poultry breeding farms might be vehicles of human fungal infections. | [74] | |
| Bacterial communities in PM2.5 and PM10 in broiler houses at different broiler growth stages in spring | China | Poultry farms (n = 3) | Animal health | Bacteria | Air samples (n = 3) | Active methods (ZR-3920 environmental air particulate matter sampler using 9 cm Tissuquartz™ filters, Pall, Port Washington, NY, USA, flow rate = 100 L/min, 48 h) | Molecular tools (PCR; 16s rRNA gene sequencing | Results revealed that PM2.5, PM10 airborne microbes gradually increased during the broiler growth cycle in poultry houses. Additionally, some potential or opportunistic pathogens were found in the broiler houses at different growth stages | [57] | |
| Size-related bacterial diversity and tetracycline resistance gene abundance in the air of concentrated poultry feeding operations | China | Poultry farms (n = 8) | Occupational and animal health | Bacteria | Air samples (outside the office; inside/outside the layer house; inside/outside the broiler house n = 5) | Active methods (eight-stage non-viable Andersen samplers coupled with quartz fiber, flow rate = 28.3 L/min, 48 h) | Molecular tools (qPCR; 16s rRNA, tetL, tetW, and E. coli gene sequencing | The richness of biological genera in the urban atmospheric environment was lower than in concentrated poultry feeding operations. The bacterial lineages of bioaerosols present in the seven size stages for layers clustered apart from those for broilers, suggesting that the type of poultry house is a more important factor than the particle size in shaping the microbial communities. Results suggest that bioaerosols containing antibiotic resistance genes and potential airborne pathogens from animal feeding operations can be efficiently transferred to the nearby environment. | [54] | |
| Slaughterhouses Fungal Burden Assessment: A Contribution for the Pursuit of a Better Assessment Strategy | Portugal | Poultry (n = 1), swine/bovine (n = 1), and large animal slaughterhouses (n = 1) | Occupational health | Fungi | Air samples: (poultry n = 6; swine/bovine n = 6; large animal slaughterhouses n = 6) Surface samples (poultry floor n = 6; swine/bovine walls n = 6; animal floor slaughterhouses n = 6) | Active methods (impaction method, Millipore air tester, Millipore, flow rate = 140 L/min) Passive methods (swabs) | Culture-based methods Molecular tools (qPCR) | Poultry and swine/bovine slaughterhouses each presented two sampling sites that surpass the guideline of 150 CFU/m3. A. fumigatus complex was identified through molecular tools in six sampling sites. Results evidence indicators that are representative of harmful fungal contamination in these settings. | [39] | |
| Occupational exposure to airborne microorganisms, endotoxins and β-glucans in poultry houses at different stages of the production cycle | Poland | Poultry farms (n = 3) | Occupational health | Bacteria Fungi, endotoxins, and β-glucans | Air samples (different stages of chicken production cycle, n = 3) | Active methods (six-stage Andersen impactor Model 10–710, Andersen Instruments, Atlanta, GA, USA, flow rate = 28.3 L/min, 0.5 to 2 min; Harvard impactors with 37 mm Teflon filters with 1 μm pore size, SKC Ltd., measurements of PM10, flow rate = 10 L/min, 4 h; filter samplers, button aerosol sampler, SKC Ltd., Eighty Four, PA, USA, clipped onto a worker’s collar. Collected on gelatin filters of 25 mm with a pore size of 3 μm, SKC Ltd., flow rate = 4 L/min, 30 min; Harvard impactors with 37 mm Teflon filters with 1 μm pore size, SKC Ltd.,measurements of PM10, flow rate =10 L/min, 4 h) | Culture-based methods LAL Quantitative kinetic Glucatell assay (β-glucans) | The level of PM10 in poultry facilities did not exceed 4.5 mg/m3. After the flock entered the clean house, the level of endotoxins and β-glucans increased from below detection limit to 8364 ng/m3 and from 0.8 ng/m3 to 6886 ng/m3, respectively. The results show that professional activities in poultry farms are associated with constant exposure to bioaerosol, which may pose a health hazard to workers. In addition, it was found that workers’ exposure to airborne microorganisms increased with consecutive stages of the chicken production cycle. | [31] | |
| Fungal Contamination of Poultry Litter: A Public Health Problem | Portugal | Poultry farms (n = 7) | Occupational health | Fungi | Air samples (n = 27) Litter collection (fresh n = 7, aged n = 14; 10 gr) | Active methods (impaction method) Passive methods (material collection) | Culture-based methods Molecular tools (qPCR) | A significant positive correlation was found between litter fungal contamination (CFU/g) and air fungal contamination (CFU/m3). Spreading of poultry litter in agricultural fields is a potential public health concern, since keratinophilic (Scopulariopsis and Fusarium genera) as well as toxigenic fungi (Aspergillus, Fusarium, and Penicillium genera) were isolated. | [38] | |
| The variability of bacterial aerosol in poultry houses depending on selected factors | Poland | Poultry farms (n = 5) | Occupational health | Bacteria | Air samples (n = 11) | Active methods (filtration method using the GilAir 5 pump, Sensidyne, Clearwater, Florida, USA; open-faced aerosol sampler, Two-Met, Zgierz, Poland, with a GF/A glass microfiber filter, Whatman International Ltd., Maidstone, Kent, UK, with a pore size of 1.6 μm, flow rate = 2 L/min, 4–6 h) | Culture-based methods | The lowest concentrations of total bacteria were obtained in those buildings where one-day-old chickens were kept. It was shown that for most of the investigated livestock premises the total bacterial concentrations exceeded the reference value of 1.0 × 105 cfu/m3. Furthermore, pathogenic microorganisms which are a potential threat to human health were found among the identified bacteria. | [30] | |
| Endotoxin concentration in poultry houses for laying hens kept in cages or in alternative housing systems | France | Poultry farms n = 21 (caged n = 8, free-cage n = 13) | Occupational health | Endotoxins | Air samples (n = 2) Personal air samples (n = 2) | Active methods (CAP 10, ARELCO, Auxerre, France, flow rate = 1 L/min, 7, 8 h; personal air samples collected in 37 mm diameter glass fiber filters with a pore size of 0–5 mm; Millipore AP4003705, St Quentin, France), aseptically placed in three-part polystyrene filter holders, Millipore M000037AO, in a constant airflow pump, SKC 224, PCTX8, ARELCO, flow rate = 1 L/min, 6 h) | LAL | The endotoxin concentrations in the ambient air, and to which workers were exposed, appeared to be high in comparison with the threshold of 50 EU/m3 over 8 h. Differences in dust and endotoxin concentrations between the cage and alternative systems may be due to the presence of litter and to the greater activity of the hens in the on-floor buildings. Effective methods to reduce worker exposure to air contaminants in laying houses still need to be developed. | [48] | |
| Culture-Independent Characterization of Bacteria and Fungi in a Poultry Bioaerosol Using Pyrosequencing: A New Approach | USA | Poultry farm | Occupational health | Bacteria Fungi | Air samples (n = 29) | Active methods (inhalable sampler, IOM, SKC Inc., Eighty Four, PA, was loaded with a 25 mm, sterile, gelatin membrane filter with a pore size of 3 μm, SKC Inc., connected to a personal sampling pump Model 210–5000, SKC Inc, flow rate = 2 L/min, 8 h) | Molecular tools (tag-encoded flexible (FLX) amplicon pyrosequencing (bTEFAP) and fungal tag-encoded flexible (FLX) amplicon pyrosequencing (fTEFAP)) | Concerning bacteria and fungi detected, 116 and 39 genera were identified, respectively. Among bacteria, Staphylococcus cohnii was present in the highest proportion (23%). The total inhalable bacteria concentration was estimated to be 7503 cells/m3. Among the fungi identified, Sagenomella sclerotialis was present in the highest proportion (37%). Aspergillus ochraceus and Penicillium janthinellum were also present in high proportions. Briefly, a limited amount of information exists on the bioaerosols present in a poultry production environment. Future work should include an expanded sampling plan and additional production sites for enhanced generalizability of the results. | [66] | |
| Air biocontamination in a variety of agricultural industry environments in Egypt: a pilot study | Egypt | Several workplaces (poultry farm, flourmill, textile and food industry) | Occupational health | Bacteria Fungi | Air samples (poultry farm n = 4; flourmill n = 8; textile n = 8 and food industry n = 2) | Active methods (liquid impinger AGI-30, Vineland, New Jersey, USA, containing 20 mL phosphate buffer, KH2PO4 0.4%, K2HPO4 1.36%, flow rate = 12.5 L/min, 15 min). Gravimetric sampler: open-faced holder with cellulose nitrate membrane filters, pore size 0.45 lm, diameter 25 mm; Whatman, Maidstone, UK, flow rate = 8 L/min, 2 h) | Culture-based methods | The highest median indoor concentration of culturable airborne bacteria (6.23 × 105 CFU/m3) was found at the occupied poultry farm. Meanwhile, the highest median indoor concentration of culturable airborne fungi (3.15 × 104 CFU/m3) was found at the flourmill site. In short, workers in Egyptian agriculture-related industries are exposed to aerosolized particulate matter and microbial concentrations. | [73] | |
| Detection of Jeotgalicoccus spp. In poultry house air | Germany | Poultry farms (n = 3) (turkey, chicken, and duck houses), duck slaughterhouse (n = 3) | Occupational health | Bacteria | Air samples (turkey n = 9; duck n = 9; chicken n = 9 farms; duck slaughterhouses n = 9) | Active methods (filtration devices, MD8 aluminum stacks; Sartorius, Germany, with polycarbonate membrane filters, Isopore ATTP 0.8 lm pore size; Millipore, for poultry farms, flow rate = 27.2 L/min, 2 h; personal air samplers, PGP/GSP-3.5; BIA, Germany, in combination with specific SG-10 (GSA) pumps, for duck farms, with polycarbonate filters, 0.8 lm pore size; 3.7 cm; Whatman, flow rate= 3.5 L/min, 8 h) | Molecular tools (16s rRNA gene sequencing) | Estimated concentrations by quantitative real-time PCR analyses revealed cell numbers between 104 and 106 of Jeotgalicoccus sp. per m−3 of air in turkey, duck, and chicken houses. These results indicated the remarkable proportion (1–39%) of total cell counts and the hitherto unknown wide distribution of Jeotgalicoccus sp. in the poultry rearing industry. | [26] | |
| Direct Detection of Salmonella Cells in the Air of Livestock Stables by Real-Time PCR | Germany | Poultry farms (broiler farm n = 2, duck farm n = 1) | Occupational health | Bacteria | Air samples (turkey n = 9; duck n = 9; chicken n = 9 farms; duck slaughterhouses n = 9) | Active methods (filtration devices, MD8 aluminum stacks; Sartorius, Germany, with polycarbonate membrane filters, Isopore ATTP 0.8 lm pore size; Millipore, for broiler farms, flow rate = 27.2 L/min, 2 h; personal air samplers, PGP/GSP-3.5; BIA, Germany, in combination with specific SG-10 (GSA) pumps, for duck farm, on polycarbonate filters, 0.8 lm pore size; 3.7 cm; Whatman, flow rate = 3.5 L/min, 8 h) | Culture based-methods Molecular tools (qPCR) | The results demonstrate airborne Salmonella sp. workplace concentrations in poultry production of up to 3.3% of 49,6-diamidino-2-phenylindole-counted total cell numbers. The risk of infection at these working places seems quite high. | [24] | |
| A case study of airborne culturable microorganisms in a poultry slaughterhouse in Styria, Austria | Austria | Poultry slaughterhouse | Occupational health | Bacteria Fungi | Air samples (Hanging area and eviscerating area, n = 2) | Active methods (Andersen six-stage viable cascade impactor, ACFM, Graseby, USA, flow rate = 28.3 L/min, 15s; impingement method, SKC biosampler, SKC, USA, flow rate = 10 L/min, 10 min) | Culture based-methods | The median concentration of airborne mesophilic bacteria was 1.7 × 106 CFU/m3 in the processing area of the “moving rail,” which is 8000 times higher than the background concentration of residential areas (approx. 210 CFU/m3). Results evidence that poultry slaughterhouse employees are exposed to high concentrations of airborne microorganisms throughout the entire work time without using a respiratory protective device. | [46] | |
| Exposure Levels of Airborne Bacteria and Fungi in Korean Swine and Poultry Sheds | Korea | Animal farms (poultry n = 4; swine farm n = 2) | Occupational health | Bacteria Fungi | Air samples (winter n = 68, summer n = 60) | Active methods (single-stage Andersen samplers with 400 0.25 mm holes, flow rate = 28.3 L/min, 0.5–2 min) | Culture based-methods | Aspergillus, Cladosporium, and Penicillium represented most of the fungi (96% and 82% in the swine sheds for winter and summer, respectively, and 69% in the poultry sheds). Many microbial concentrations exceeded the Korean indoor bioaerosol guideline of 800 CFU/m3. | [58] | |
| Airborne Fungi In Industrial Environments—Potential Agents Of Respiratory Diseases | Lithuania | Several workplaces (poultry farm, swinery, feed preparing and storing house, grain mill, wooden panel producing factory, and organic waste recycling facilities, n = 6) | Occupational health | Fungi | Air samples | Active methods (AGI-30 all glass impinger, Ace Glass Inc., Vineland, NJ, USA; cut-off 0.31 μm; air filtering through 47 mm cellulose membrane, Whatman plc, Kent, UK, pore size not specified, mounted on a plastic filter holder, flow rate = 0.001 m3/min, 15 min; Krotov 818 impactor was operated for 1 and 2 min at the flow rate = 0.025 m3/min, 1–2 min) | Culture based-methods | Thirty-one species attributed to thirteen fungal genera were isolated from the poultry house air. According to evidence, the majority of the identified fungal species found in industrial environments are characterized as allergenic and exposure to their spores may provoke adverse health effects in susceptible individuals. | [47] | |
| Air contaminants in different european farming environments | European countries (Denmark, Switzerland, Spain) | Animal farms (pig farm in Denmark, poultry farm in Switzerland, and greenhouse in Spain) | Occupational health | Bacteria Fungi Endotoxins | Air samples Personal air samples | Active methods (polycarbonate filters with a pore size of 0.4 μm and a diameter of 25 mm were placed on cellulose support pads and sealed in presterilized carbon-filled polypropylene air monitoring cassettes, Pegasus Labor, Duesseldorf, Germany, connected to portable battery-operated pumps, flow rate = 1 L/min; airborne dust (PM10) was collected on preweighed 37 mm diameter glass fiber filters fixed in threaded holders; personal air sampler, battery-operated pumps, flow rate = 3.5 L/min) | Culture based-methods Fluorescence quantification LAL | The highest total dust concentrations were found in poultry houses in Switzerland with median concentrations of 7.01 mg/m3. The highest total and active fungus concentrations were detected in poultry houses compared to pig houses and greenhouses. Additionally, bacterial concentrations were high in all animal houses. The exposure level found in this study might put the farmers at risk of respiratory diseases. | [52] | |
| Exposure assessment and lung function in pig and poultry farmers | Denmark and Switzerland | Animal farms (poultry farm, Denmark; Swine farm, Switzerland) | Occupational health | Bacteria Fungi Endotoxins | Air samples Personal air samples (poultry farmers n = 36, pig farmers n = 40) | Active methods (polycarbonate filters with a pore size of 0.4 μm and a diameter of 25 mm placed on cellulose support pads and sealed in presterilized carbon-filled polypropylene air monitoring cassettes, airflow = 1 L/min; dust was collected on preweighed 37 mm diameter glass fiber filters fixed in threaded holders, flow rate = 3.5 L/min) | Questionnaires Spirometry LAL Fluorescence quantification | Results evidence that factors related to work in the housing areas of pigs and poultry (variables of ventilation and feeding management) were significantly associated with decrements in lung function. Additionally, higher temperatures inside the pig houses were significantly negatively associated with lung function in pig farmers. Overall, lung function results were significantly associated with ventilation of the animal houses. | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.; Dias, M.; Cervantes, R.; Pena, P.; Santos, J.; Vasconcelos Pinto, M.; Viegas, C. One Health Approach to Tackle Microbial Contamination on Poultries—A Systematic Review. Toxics 2023, 11, 374. https://doi.org/10.3390/toxics11040374
Gomes B, Dias M, Cervantes R, Pena P, Santos J, Vasconcelos Pinto M, Viegas C. One Health Approach to Tackle Microbial Contamination on Poultries—A Systematic Review. Toxics. 2023; 11(4):374. https://doi.org/10.3390/toxics11040374
Chicago/Turabian StyleGomes, Bianca, Marta Dias, Renata Cervantes, Pedro Pena, Joana Santos, Marta Vasconcelos Pinto, and Carla Viegas. 2023. "One Health Approach to Tackle Microbial Contamination on Poultries—A Systematic Review" Toxics 11, no. 4: 374. https://doi.org/10.3390/toxics11040374
APA StyleGomes, B., Dias, M., Cervantes, R., Pena, P., Santos, J., Vasconcelos Pinto, M., & Viegas, C. (2023). One Health Approach to Tackle Microbial Contamination on Poultries—A Systematic Review. Toxics, 11(4), 374. https://doi.org/10.3390/toxics11040374