Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS) Coupled with an Interferometer for On-Band and Off-Band Detection of Glyoxal
Abstract
:1. Introduction
2. Materials and Methods
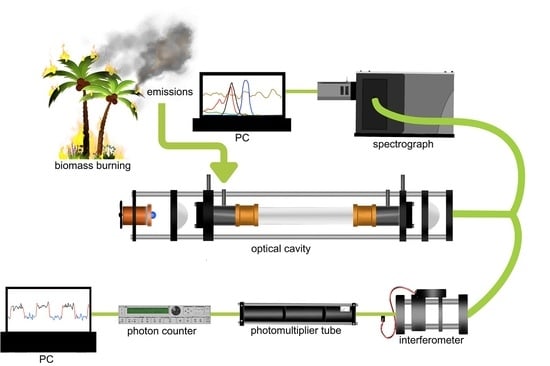
2.1. Experimental Setup
2.2. Interferometer Setup
2.3. Data Processing
2.3.1. Spectrometer Data
2.3.2. Interferometer Data
2.3.3. Error Analysis
3. Results and Discussion
3.1. Dual Detection (Spectrometer/CCD and Interferometer/PMT)
3.1.1. Spectrometer/CCD
3.1.2. Interferometer/PMT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Environmental Protection Agency. Ethanedial. 2023. Available online: https://cdxapps.epa.gov/oms-substance-registry-services/substance-details/24265 (accessed on 30 October 2023).
- Volkamer, R.; Platt, U.; Wirtz, K. Primary and secondary glyoxal formation from aromatics: Experimental evidence for the bicycloalkyl-radical pathway from benzene, toluene, and p-xylene. J. Phys. Chem. A 2001, 105, 7865–7874. [Google Scholar] [CrossRef]
- Lim, H.J.; Carlton, A.G.; Turpin, B.J. Isoprene forms secondary organic aerosol through cloud processing: Model simulations. Environ. Sci. Technol. 2005, 39, 4441–4446. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.M.; Jacob, D.J.; Wittrock, F.; Burrows, J.P.; Vrekoussis, M.; Henze, D.K. Global budgets of atmospheric glyoxal and methylglyoxal, and implications for formation of secondary organic aerosols. J. Geophys. Res.-Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Wittrock, F.; Richter, A.; Oetjen, H.; Burrows, J.P.; Kanakidou, M.; Myriokefalitakis, S.; Volkamer, R.; Beirle, S.; Platt, U.; Wagner, T. Simultaneous global observations of glyoxal and formaldehyde from space. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Ryan, R.G.; Rhodes, S.; Tully, M.; Schofield, R. Surface ozone exceedances in Melbourne, Australia are shown to be under NOx control, as demonstrated using formaldehyde: NO2 and glyoxal:formaldehyde ratios. Sci. Total Environ. 2020, 749, 141460. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Wolfe, G.M.; Min, K.E.; Brown, S.S.; Miller, C.C.; Jacob, D.J.; deGouw, J.A.; Graus, M.; Hanisco, T.F.; Holloway, J.; et al. Reassessing the ratio of glyoxal to formaldehyde as an indicator of hydrocarbon precursor speciation. Atmos. Chem. Phys. 2015, 15, 7571–7583. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, C.; Su, W.J.; Hu, Q.H.; Zhang, C.X.; Liu, H.R.; Yin, H. Identification of volatile organic compound emissions from anthropogenic and biogenic sources based on satellite observation of formaldehyde and glyoxal. Sci. Total Environ. 2023, 859, 159997. [Google Scholar] [CrossRef] [PubMed]
- DiGangi, J.P.; Henry, S.B.; Kammrath, A.; Boyle, E.S.; Kaser, L.; Schnitzhofer, R.; Graus, M.; Turnipseed, A.; Park, J.H.; Weber, R.J.; et al. Observations of glyoxal and formaldehyde as metrics for the anthropogenic impact on rural photochemistry. Atmos. Chem. Phys. 2012, 12, 9529–9543. [Google Scholar] [CrossRef]
- Vrekoussis, M.; Wittrock, F.; Richter, A.; Burrows, J.P. GOME-2 observations of oxygenated VOCs: What can we learn from the ratio glyoxal to formaldehyde on a global scale? Atmos. Chem. Phys. 2010, 10, 10145–10160. [Google Scholar] [CrossRef]
- Hong, Q.Q.; Liu, C.; Hu, Q.H.; Zhang, Y.L.; Xing, C.Z.; Ou, J.P.; Tan, W.; Liu, H.R.; Huang, X.Q.; Wu, Z.F. Vertical distribution and temporal evolution of formaldehyde and glyoxal derived from MAX-DOAS observations: The indicative role of VOC sources. J. Environ. Sci. 2022, 122, 92–104. [Google Scholar] [CrossRef]
- Liu, J.W.; Li, X.; Tan, Z.F.; Wang, W.J.; Yang, Y.M.; Zhu, Y.; Yang, S.D.; Song, M.D.; Chen, S.Y.; Wang, H.C.; et al. Assessing the Ratios of Formaldehyde and Glyoxal to NO2 as Indicators of O-3-NOx-VOC Sensitivity. Environ. Sci. Technol. 2021, 55, 10935–10945. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.J.; Gong, D.C.; Ding, Y.Z.; Lin, Y.J.; Wang, H.; Ding, H.; Wu, G.C.; He, C.Q.; Zhou, L.; Liu, S.C.; et al. Elevated levels of glyoxal and methylglyoxal at a remote mountain site in southern China: Prompt in-situ formation combined with strong regional transport. Sci. Total Environ. 2019, 672, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.H.; Wang, S.X.; Ying, Q.; Duan, L.; Xing, J.; Cao, J.Y.; Wu, D.; Li, X.X.; Xing, C.Z.; Yan, X.; et al. Importance of Wintertime Anthropogenic Glyoxal and Methylglyoxal Emissions in Beijing and Implications for Secondary Organic Aerosol Formation in Megacities. Environ. Sci. Technol. 2020, 54, 11809–11817. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, X.M.; Wen, S.; Herrmann, H.; Yang, W.Q.; Huang, X.Y.; Zhang, Z.; Huang, Z.H.; He, Q.F.; George, C. On-road vehicle emissions of glyoxal and methylglyoxal from tunnel tests in urban Guangzhou, China. Atmos. Environ. 2016, 127, 55–60. [Google Scholar] [CrossRef]
- Grosjean, D.; Grosjean, E.; Gertler, A.W. On-road emissions of carbonyls from light-duty and heavy-duty vehicles. Environ. Sci. Technol. 2001, 35, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kean, A.J.; Grosjean, E.; Grosjean, D.; Harley, R.A. On-road measurement of carbonyls in California light-duty vehicle emissions. Environ. Sci. Technol. 2001, 35, 4198–4204. [Google Scholar] [CrossRef]
- Zarzana, K.J.; Min, K.E.; Washenfelder, R.A.; Kaiser, J.; Krawiec-Thayer, M.; Peischl, J.; Neuman, J.A.; Nowak, J.B.; Wagner, N.L.; Dube, W.P.; et al. Emissions of Glyoxal and Other Carbonyl Compounds from Agricultural Biomass Burning Plumes Sampled by Aircraft. Environ. Sci. Technol. 2017, 51, 11761–11770. [Google Scholar] [CrossRef]
- Zarzana, K.J.; Selimovic, V.; Koss, A.R.; Sekimoto, K.; Coggon, M.M.; Yuan, B.; Dube, W.P.; Yokelson, R.J.; Warneke, C.; de Gouw, J.A.; et al. Primary emissions of glyoxal and methylglyoxal from laboratory measurements of open biomass burning. Atmos. Chem. Phys. 2018, 18, 15451–15470. [Google Scholar] [CrossRef]
- Hays, M.D.; Geron, C.D.; Linna, K.J.; Smith, N.D.; Schauer, J.J. Speciation of gas-phase and fine particle emissions from burning of foliar fuels. Environ. Sci. Technol. 2002, 36, 2281–2295. [Google Scholar] [CrossRef]
- Vrekoussis, M.; Wittrock, F.; Richter, A.; Burrows, J.P. Temporal and spatial variability of glyoxal as observed from space. Atmos. Chem. Phys. 2009, 9, 4485–4504. [Google Scholar] [CrossRef]
- Edwards, P.M.; Brown, S.S.; Roberts, J.M.; Ahmadov, R.; Banta, R.M.; deGouw, J.A.; Dube, W.P.; Field, R.A.; Flynn, J.H.; Gilman, J.B.; et al. High winter ozone pollution from carbonyl photolysis in an oil and gas basin. Nature 2014, 514, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, R.J.; Yang, L.; Dai, W.T.; Ni, H.Y.; Gong, Y.Q.; Guo, J.; Zhong, H.B.; Lin, C.S.; Xu, W. Direct emissions of particulate glyoxal and methylglyoxal from biomass burning and coal combustion. Sci. Total Environ. 2023, 862, 160757. [Google Scholar] [CrossRef] [PubMed]
- Thalman, R.; Volkamer, R. Inherent calibration of a blue LED-CE-DOAS instrument to measure iodine oxide, glyoxal, methyl glyoxal, nitrogen dioxide, water vapour and aerosol extinction in open cavity mode. Atmos. Meas. Tech. 2010, 3, 1797–1814. [Google Scholar] [CrossRef]
- Fang, B.; Zhao, W.X.; Xu, X.Z.; Zhou, J.C.; Ma, X.; Wang, S.; Zhang, W.J.; Venables, D.S.; Chen, W.D. Portable broadband cavity-enhanced spectrometer utilizing Kalman filtering: Application to real-time, in situ monitoring of glyoxal and nitrogen dioxide. Opt. Express 2017, 25, 26910–26922. [Google Scholar] [CrossRef] [PubMed]
- Washenfelder, R.A.; Langford, A.O.; Fuchs, H.; Brown, S.S. Measurement of glyoxal using an incoherent broadband cavity enhanced absorption spectrometer. Atmos. Chem. Phys. 2008, 8, 7779–7793. [Google Scholar] [CrossRef]
- Ball, S.M.; Jones, R.L. Broad-band cavity ring-down spectroscopy. Chem. Rev. 2003, 103, 5239–5262. [Google Scholar] [CrossRef] [PubMed]
- Ruth, A.A.; Orphal, J.; Fiedler, S.E. Fourier-transform cavity-enhanced absorption spectroscopy using an incoherent broadband light source. Appl. Opt. 2007, 46, 3611–3616. [Google Scholar] [CrossRef]
- O’Keefe, A. Integrated cavity output analysis of ultra-weak absorption. Chem. Phys. Lett. 1998, 293, 331–336. [Google Scholar] [CrossRef]
- Gherman, T.; Romanini, D. Mode-locked cavity-enhanced absorption spectroscopy. Opt. Express 2002, 10, 1033–1042. [Google Scholar] [CrossRef]
- Gianluca Gagliardi, H.-P.L. Cavity-Enhanced Spectroscopy and Sensing; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Giel Berden, R.E. Cavity Ring-Down Spectroscopy; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lehmann, K.K.; Romanini, D. The superposition principle and cavity ring-down spectroscopy. J. Chem. Phys. 1996, 105, 10263–10277. [Google Scholar] [CrossRef]
- Fuchs, C.; Kuhn, J.; Bobrowski, N.; Platt, U. Quantitative imaging of volcanic SO2 plumes using Fabry-Perot interferometer correlation spectroscopy. Atmos. Meas. Tech. 2021, 14, 295–307. [Google Scholar] [CrossRef]
- Kuhn, J.; Bobrowski, N.; Wagner, T.; Platt, U. Mobile and high-spectral-resolution Fabry-Perot interferometer spectrographs for atmospheric remote sensing. Atmos. Meas. Tech. 2021, 14, 7873–7892. [Google Scholar] [CrossRef]
- Flowerday, C.E.; Bhardwaj, N.; Thalman, R.; Asplund, M.C.; Sevy, E.T.; Hansen, J.C. Absorption cross-sections for the 5th and 6th vibrational overtones in a series of short chained alcohols using incoherent broadband cavity enhanced-absorption spectroscopy (IBBCEAS). J. Mol. Spectrosc. 2023, 392, 111746. [Google Scholar] [CrossRef]
- Thalman, R.; Bhardwaj, N.; Flowerday, C.E.; Hansen, J.C. Detection of Sulfur Dioxide by Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS). Sensors 2022, 22, 2626. [Google Scholar] [CrossRef]
- Thalman, R.; Zarzana, K.J.; Tolbert, M.A.; Volkamer, R. Rayleigh scattering cross-section measurements of nitrogen, argon, oxygen and air. J. Quant. Spectrosc. Radiat. Transf. 2014, 147, 171–177. [Google Scholar] [CrossRef]
- Volkamer, R.; Spietz, P.; Burrows, J.; Platt, U. High-resolution absorption cross-section of glyoxal in the UV-vis and IR spectral ranges. J. Photochem. Photobiol. A Chem. 2005, 172, 35–46. [Google Scholar] [CrossRef]
- Meller, R.; Raber, W.; Crowley, J.N.; Jenkin, M.E.; Moortgat, G.K. The UV-visible absorption-spectrum of methylglyoxal. J. Photochem. Photobiol. A Chem. 1991, 62, 163–171. [Google Scholar] [CrossRef]
- Vandaele, A.C.; Hermans, C.; Fally, S.; Carleer, M.; Colin, R.; Mérienne, M.F.; Jenouvrier, A.; Coquart, B. High-resolution Fourier transform measurement of the NO2 visible and near-infrared absorption cross sections: Temperature and pressure effects. J. Geophys. Res.-Atmos. 2002, 107, ACH 3-1–ACH 3-12. [Google Scholar] [CrossRef]
- Thalman, R.; Baeza-Romero, M.T.; Ball, S.M.; Borrás, E.; Daniels, M.J.S.; Goodall, I.C.A.; Henry, S.B.; Karl, T.; Keutsch, F.N.; Kim, S.; et al. Instrument intercomparison of glyoxal, methyl glyoxal and NO2 under simulated atmospheric conditions. Atmos. Meas. Tech. 2015, 8, 1835–1862. [Google Scholar] [CrossRef]
- Chandran, S.; Puthukkudy, A.; Varma, R. Dual-wavelength dual-cavity spectrometer for NO2 detection in the presence of aerosol interference. Appl. Phys. B-Lasers Opt. 2017, 123, 213. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kelsch, A.; Eatough, D.J.; Thalman, R.; Daher, N.; Kelly, K.; Jaramillo, I.C.; Hansen, J.C. Sources of Formaldehyde in Bountiful, Utah. Atmosphere 2021, 12, 375. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flowerday, C.E.; Thalman, R.; Asplund, M.C.; Hansen, J.C. Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS) Coupled with an Interferometer for On-Band and Off-Band Detection of Glyoxal. Toxics 2024, 12, 26. https://doi.org/10.3390/toxics12010026
Flowerday CE, Thalman R, Asplund MC, Hansen JC. Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS) Coupled with an Interferometer for On-Band and Off-Band Detection of Glyoxal. Toxics. 2024; 12(1):26. https://doi.org/10.3390/toxics12010026
Chicago/Turabian StyleFlowerday, Callum E., Ryan Thalman, Matthew C. Asplund, and Jaron C. Hansen. 2024. "Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS) Coupled with an Interferometer for On-Band and Off-Band Detection of Glyoxal" Toxics 12, no. 1: 26. https://doi.org/10.3390/toxics12010026
APA StyleFlowerday, C. E., Thalman, R., Asplund, M. C., & Hansen, J. C. (2024). Broadband Cavity-Enhanced Absorption Spectroscopy (BBCEAS) Coupled with an Interferometer for On-Band and Off-Band Detection of Glyoxal. Toxics, 12(1), 26. https://doi.org/10.3390/toxics12010026