Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders
Abstract
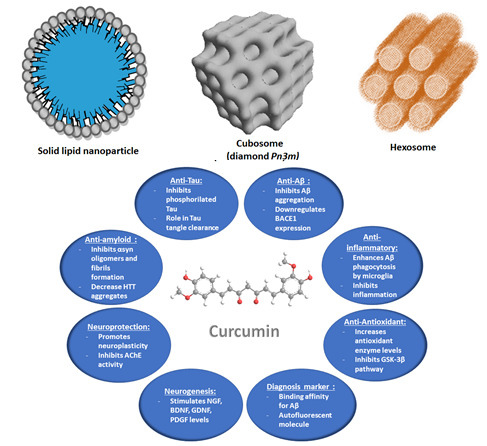
:1. Introduction
2. Risk Factors for Neurodegenerative Disorders
3. Curcumin Potential for Neuroprotection against Neurodegenerative Diseases
3.1. In Vitro and In Vivo Studies of Curcumin Properties in Neurodegenerative Disease Models
3.2. Clinical Trials and Curcumin Limits
4. Nanocarrier-Mediated Curcumin Delivery
4.1. Curcumin Delivery by Polymeric Nanoparticles
4.2. Curcumin Delivery by Lipid Nanoparticles
4.2.1. Solid Lipid Nanoparticles (SLNPs) and Nanostructured Lipid Carriers (NLCs)
4.2.2. Liposomes
4.2.3. Liquid Crystalline Nanoparticles (LCNPs) with Internal Structure
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid βeta |
| ACh | Acetylcholine |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ApoE | Apolipoprotein E |
| APP | Amyloid beta precursor protein |
| ARE | Antioxidant response element |
| BBB | Brain blood barrier |
| BDNF | Brain derived neurotrophic factor |
| Ca2+ | Calcium ion |
| CAT | Catalase |
| CHCHD2 | Coiled-coil-helix-coiled-coil-helix domain 2 |
| C9ORF72 | Chromosome 9 open reading frame 72 |
| COMT | Catechol-O-methyltransferase |
| CREB | cAMP (Cyclic adenosine monophosphate response) element-binding protein |
| CU | Curcumin |
| CYP | Cytochrome P450 |
| DARPP | Dopamine and adenosine 3′,5′-monophosphate-regulated phosphoprotein |
| DHA | Docosahexaenoic acid |
| DNA | Deoxyribonucleic acid acid |
| DNAJC13 | DNA J heat shock protein family (Hsp40) member C13 |
| DSPE | Distearoy phosphatidylethanolamine |
| EIF4G1 | Eukaryotic translation initiation factor 4 gamma 1 |
| EMA | European medicines agency |
| ERK | Extracellular signal regulated kinase |
| FDA | Food and drug administration |
| FUS | RNA binding protein Fused in Sarcoma |
| GBA | Glucocerebrosidase |
| GRAS | Generally recognized as safe |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| HEWL | Hen Egg White Lysozyme |
| HD | Huntington disease |
| H2O2 | Hydrogen peroxide |
| HTT | Huntingtin |
| IL-6 | Interleukin 6 |
| iNOS | induced nitric oxide synthase |
| IV | intravenous |
| JNK | Jun N-terminal kinase |
| LCNs | Liquid crystalline nanocarriers |
| LDL | Low density lipoprotein |
| Lf | Lactoferrin |
| LPS | Lipopolysaccharide |
| LRRK1 | Leucine-rich repeat kinase 1 |
| LUV | Large unilamellar vesicles |
| MAO-B | Monoamine oxidase type B |
| MDA | Malondialdehyde |
| MLV | Multilamellar vesicles |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MMSE | Mini Mental State Examination |
| mRNA | Messenger Ribonucleic Acid |
| NF-kb | Nuclear Factor Kappa Beta |
| NGF | Nerve growth factor |
| NLC | Nanostructured lipid carriers |
| NO | Nitric oxide |
| NPs | nanoparticles |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OHDA | 6 Hydroxydopamine |
| OPTN | Optineurin |
| PD | Parkinson disease |
| PEG | Polyethylene glycol |
| PINK1 | PTEN-induced putative kinase 1 |
| PLGA | Poly (lactic-co-glycolic acid) |
| PRKN | Parkin |
| PSEN | Presenilin |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SLCP | Solid lipid curcumin nanoparticles |
| SLN | Solid lipid nanoparticles |
| SOD1 | Superoxide dismutase 1 |
| SNCA | Synuclein alpha |
| SUV | Small unilamellar vesicles |
| TARDBP | TAR DNA binding protein (TDP-43) |
| TREG | T regulatory cell |
| TRPME | Transient Receptor Potential Mucolipin-1 Expression |
| TrkB | Tropomyosin receptor kinase B |
| UBQLN2 | Ubiquitin 2 |
| UCH | Ubiquitin carboxy-terminal hydrolase |
| VPS35 | Vascular protein sorting |
| WGA | Wheat-germ agglutinin |
References
- Brookmeyer, R.; Abdalla, N.; Kawas, C.H.; Corrada, M.M. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018, 14, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wakasaya, Y.; Kawarabayashi, T.; Watanabe, M.; Yamamoto-Watanabe, Y.; Takamura, A.; Kurata, T.; Murakami, T.; Abe, K.; Yamada, K.; Wakabayashi, K. Factors responsible for neurofibrillary tangles and neuronal cell losses in tauopathy. J. Neurosci. Res. 2011, 89, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Stoothoff, W.H.; Johnson, G.V. Tau phosphorylation: Physiological and pathological consequences. Biochim. Biophys. Acta 2005, 1739, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.M.; Li, X.M.; Li, F.; Wu, J.J.; Kong, L.Y.; Wang, X.B. Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer’s disease based on the fusion of donepezil and melatonin. Bioorgan. Med. Chem. 2016, 24, 4324–4338. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Morihara, T.; Lim, G.P.; Yang, F.; Begum, A.; Frautschy, S.A. NSAID and antioxidant prevention of Alzheimer’s disease: Lessons from in vitro and animal models. Ann. N. Y. Acad. Sci. 2004, 1035, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259–280. [Google Scholar] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Landles, C.; Bates, G.P. Huntingtin and the molecular pathogenesis of Huntington’s disease: Fourth in molecular medicine review series. EMBO Rep. 2004, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, Y.; Qian, S.; Chang, W.; Wang, L.; Fan, D. Amyotrophic lateral sclerosis in Beijing: Epidemiologic features and prognosis from 2010 to 2015. Brain Behav. 2018, 19, e01131. [Google Scholar] [CrossRef] [PubMed]
- Glajch, E.; Ferraiuolo, L.; Mueller, K.A.; Stopford, M.J.; Prabhkar, V.; Gravanis, A.; Shaw, P.J.; Sadri-Vakili, G.S. Microneurotrophins improve survival in motor neuron-astrocyte co-cultures but co cot improve disease phenotypes in a mutant SOD1 mouse model of amyotrophic lateral sclerosis. PLoS ONE. 2016, 10, e0164103. [Google Scholar]
- Varinderpal, S.D.; Michael, F. Mutations that affect mitochondrial functions and their association with neurodegenerative diseases. Mutat Res. Rev. Mutat. Res. 2014, 759, 1–13. [Google Scholar]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Suna, Q.; Chena, S.B. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Progr. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Beal, M.F. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic. Biol. Med. 2011, 51, 1014–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Zhou, J.; Chen, X.; Lou, Y.; Liu, D.; Zou, X.; Yang, B.; Yin, Y.; Pan, Y. Quantitative proteomics study of the neuroprotective effects of B12 on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Sci. Rep. 2016, 6, 22635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onyango, I.G.; Khan, S.M.; Bennett, J.P. Mitochondria in the pathophysiology of Alzheimer’s and Parkinson’s diseases. Front. Biosci. 2017, 22, 854–872. [Google Scholar] [CrossRef]
- Ankarcronaa, M.; Mangialascheb, F.; Winblada, B. Thinking Alzheimer’s disease therapy: Are mitochondria the key? J. Alzheimers Dis. 2010, 20 (Suppl. 2), S579–S590. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Stahon, K.E.; Bastian, C.; Griffith, S.; Kidd, G.J.; Brunet, S.; Baltan, S. Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J. Neurosci. 2016, 36, 9990–10001. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Nakamura, T.; Lipton, S.A. Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol. Life Sci 2010, 67, 3435–3447. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Su, C.; Qiao, C.; Bian, Y.; Ding, J.; Hu, G. Metformin prevents dopaminergic neuron death in MPTP/P-induced mouse model of Parkinson’s disease via autophagy and mitochondrial ROS clearance. Int. J. Neuropsychopharmacol. 2016, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zádori, D.; Klivényi, P.; Szalárdy, L.; Fülöp, F.; Toldi, J.; Vécsei, L. Mitochondrial disturbances, excitotoxicity, neuroinflammation and kynurenines: Novel therapeutic strategies for neurodegenerative disorders. J. Neurol. Sci. 2012, 322, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Géral, C.; Angelova, A.; Lesieur, S. From molecular to nanotechnology strategies for delivery of neurotrophins: Emphasis on brain-derived neurotrophic factor (BDNF). Pharmaceutics 2013, 5, 127–167. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Angelov, B.; Drechsler, M.; Lesieur, S. Neurotrophin delivery using nanotechnology. Drug Discov. Today 2013, 18, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. 7,8-Dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2012, 37, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances. Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Hadadi, Z.; Ahmadi, M.A. Review of the anti-oxidation, anti-inflammatory and anti-tumor properties of curcumin. Tradit. Integr. Med. 2017, 2, 188–195. [Google Scholar]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Brondino, N.; Re, S.; Boldrini, A.; Cuccomarino, A.; Lanati, N.; Barale, F.; Politi, P. Curcumin as a therapeutic agent in dementia: A mini systematic review of human studies. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Dunbar, G. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018, 31, 19. [Google Scholar] [CrossRef] [PubMed]
- Haan, J.D.; Morrema, T.H.J.; Rozemuller, A.J.; Bouwman, F.H.; Hoozemans, J.J.M. Different curcumin forms selectively bind fibrillar amyloid beta in post mortem Alzheimer’s disease brains: Implications for in-vivo diagnostics. Acta Neuropathol. Commun. 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Borana, M.S.; Chaudhary, A.P. Understanding curcumin-induced modulation of protein aggregation. Int. J. Biol. Macromol. 2017, 100, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Dhiman, M.; Mittal, S.; Mantha, A.K. Curcumin revitalizes Amyloid beta (25-35)-induced and organophosphate pesticides pestered neurotoxicity in SH-SY5Y and IMR-32 cells via activation of APE1 and Nrf2. Metab. Brain Dis. 2017, 32, 2045–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Cheng, X.; Lian, Y.J.; Xu, H.L.; Zeng, Z.S.; Zhu, H.C. Curcumin exerts effects on the pathophysiology of Alzheimer’s disease by regulating PI(3,5)P2 and transient receptor potential mucolipin-1 expression. Front. Neurol. 2017, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, L.; Batool, Z.; Sadir, S.; Rafiq, S.; Shahzad, S.; Perveen, T.; Haider, S. Naringenin-induced enhanced antioxidant defense system meliorates cholinergic neurotransmission and consolidates memory in male rats. Life Sci. 2018, 194, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Vuu, M.D.; Huynh, M.A.; Yamaguchi, M.; Tran, L.T.; Dang, T.P.T. Curcumin effectively rescued Parkinson’s disease-like phenotypes in a novel drosophila melanogaster model with dUCH Knockdown. Oxid. Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Ju, B.; Zhang, Y.Z.; Yin, H.L.; Liu, Y.J.; Wang, S.S.; Zeng, Z.L.; Yang, X.P.; Wang, H.T.; Li, J.F. Protective effect of curcumin against oxidative stress-induced injury in rats with Parkinson’s disease through the Wnt/β-catenin signaling pathway. Cell. Physiol. Biochem. 2017, 43, 2226–2241. [Google Scholar] [CrossRef] [PubMed]
- Snigdha, D.M.; Surjyanarayan, M.; Jayvadan, P. Intranasal mucoadhesive microemulsion for neuroprotective effect of curcumin in mPTP induced Parkinson model. Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef]
- Hickey, M.A.; Zhu, C.; Medvedeva, V.; Lerner, R.P.; Patassini, S.; Franich, N.R.; Maiti, P.; Frautschy, S.A.; Zeitlin, S.; Levine, M.S.; et al. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol. Neurodegener. 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Sevastre-Berghian, A.C.; Făgărăsan, V.; Toma, V.A.; Bâldea, I.; Olteanu, D.; Moldovan, R.; Decea, N.; Filip, G.A.; Clichici, S.V. Curcumin reverses the diazepam-induced cognitive impairment by modulation of oxidative stress and ERK 1/2/NF-κB pathway in brain. Oxid. Med. Cell. Longev. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Motaghinejad, M.; Motevalian, M.; Fatima, S.; Hashemi, H.; Gholami, M. Curcumin confers neuroprotection against alcohol-induced hippocampal neurodegeneration via CREB-BDNF pathway in rats. Biomed. Pharmacother. 2017, 87, 721–740. [Google Scholar] [CrossRef] [PubMed]
- Motaghinejad, M.; Motevalian, M.; Fatima, S.; Faraji, F.; Mozaffari, S. The neuroprotective effect of curcumin against nicotine-induced neurotoxicity is mediated by CREB-BDNF signaling pathway. Neurochem. Res. 2017, 42, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hishikawa, N.; Takahashi, Y.; Amakusa, Y.; Tanno, Y.; Tuji, Y.; Niwa, H.; Murakami, N.; Krishna, U.K. Effects of turmeric on Alzheimer’s disease with behavioral and psychological symptoms of dementia. AYU 2012, 33, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Giacomo, S. Curcumin and resveratrol in the management of cognitive disorders: What is the clinical evidence? Molecules 2016, 21, 1243. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.R.; Jones, D.J.; Orr, S.; Coughtrie, M.W.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomark. Prev. 2002, 11, 105–111. [Google Scholar]
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J. Agric. Food. Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-applied curcumin for different diseases therapy. Biomed. Res. Int. 2014, 2014, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Modi, G.; Pillay, V.; Choonara, Y.E. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann. N. Y. Acad. Sci. 2010, 1184, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Sahni, J.K.; Doggui, S.; Ali, J.; Baboota, S.; Dao, L.; Ramassamy, C. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J. Control. Release 2011, 152, 208–231. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Bondì, M.L.; Pitarresi, G.; Cavallaro, G. Nanoparticulate systems for drug delivery and targeting to the central nervous system. CNS Neurosci. Ther. 2011, 17, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Gremiao, D.; Palmira, M.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Sun, X.; Punj, V.; Sriramoju, B.; Mohan, R.R.; Zhou, S.F.; Chauhan, A.; Kanwar, R.K. Nanoparticles in the treatment and diagnosis of neurological disorders: Untamed dragon with fire power to heal. Nanomedicine 2012, 8, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Roney, C.; Kulkarni, P.; Arora, V.; Antich, P.; Bonte, F.; Wu, A.; Mallikarjuana, N.N.; Manohar, S.; Liang, H.F.; Kulkarni, A.R.; et al. Targeted nanoparticles for drug delivery through the blood–brain barrier for Alzheimer’s disease. J. Control. Release 2005, 108, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Modi, G.; Pillay, V.; Choonara, Y.E.; Ndesendo, V.M.K.; Du Toit, L.C.; Naidoo, D. Nanotechnological applications for the treatment of neurodegenerative disorders. Progr. Neurobiol. 2009, 4, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A.; Lai, R.; Samuel, J. Micelles of poly(ethyleneoxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. A 2008, 86, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, J.; Shi, K.; Huang, Q. Structure of modified epsilon-polylysine micelles and their application in improving cellular antioxidant activity of curcuminoids. Food Funct. 2011, 2, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of novel biodegradable methoxypoly (ethylene glycol)-zein micelles for effective delivery of curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Doggui, S.; Sahni, J.K.; Arseneault, M.; Dao, L.; Ramassamy, C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J. Alzheimers Dis. 2012, 30, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed]
- Paka, G.D.; Doggui, S.; Zaghmi, A.; Safar, R.; Dao, L.; Reisch, A.; Klymchenko, A.; Roullin, V.G.; Joubert, O.; Ramassamy, C. Neuronal uptake and neuroprotective properties of curcumin-loaded nanoparticles on SK-N-SH cell line: Role of poly(lactide-coglycolide) polymeric matrix composition. Mol. Pharm. 2016, 13, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Paka, G.D.; Ramassamy, C. Optimization of curcumin-loaded PEG-PLGA nanoparticles by GSH functionalization: Investigation of the internalization pathway in neuronal cells. Mol. Pharm. 2017, 14, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.M.; Jan, W.C.; Chien, C.F.; Lee, W.C.; Lin, L.C.; Tsai, T.H. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011, 127, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood–brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. ApoE3mediated poly (butyl) cyanoacrylate nanoparticles containing curcumin: Study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol. Pharm. 2010, 7, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Le Droumaguet, B.; Nicolas, J.; Brambilla, D.; Mura, S.; Maksimenko, A.; DeKimpe, L.; Salvati, E.; Zona, C.; Airoldi, C.; Canovi, M.; et al. Versatile and efficient targeting using a single nanoparticulate platform: Application to cancer and Alzheimer’s disease. ACS Nano 2012, 6, 5866–5879. [Google Scholar] [CrossRef] [PubMed]
- Ameruoso, A.; Palomba, R.; Palange, A.; Cervadoro, A.; Lee, A.; Di Mascolo, D.; Decuzzi, P. Ameliorating amyloid-β fibrils triggered inflammation via curcumin-loaded polymeric nanoconstructs. Front. Immunol. 2017, 8, 1411. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Bisht, S.; Maitra, A.; Maitra, A.; Lahiri, D.K. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurcTM) in the neuronal cell culture and animal model: Implications for Alzheimer’s disease. J. Alzheimers Dis. 2011, 23, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Jain, K.; Gowthamarajan, K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf. B Biointerfaces 2014, 113, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Hagl, S.; Kocher, A.; Schiborr, C.; Kolesova, N.; Frank, J.; Eckert, G.P. Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice—Impact on bioavailability. Neurochem. Int. 2015, 89, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; WasiKhan, S.B.R.; Naqvi, A.H. Synthesis of alginate-curcumin nanocomposite and its protective role in transgenic drosophila model of Parkinson’s disease. ISRN Pharmacol. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.; Muppu, S.K.; Chopra, K.; Kaur, I.P. Curcumin loaded solid lipid nanoparticles: An efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur. J. Pharm. Biopharm. 2013, 85, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kakkara, V.; Mishrab, A.K.; Chuttanib, K.; Kaura, I.P. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. Int. J. Pharm. 2013, 448, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Dunbar, G.L. Comparative neuroprotective effects of dietary curcumin and solid lipid curcumin particles in cultured mouse neuroblastoma cells after exposure to Abeta42. Int. J. Alzheimers Dis. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Hall, T.C.; Paladugu, L.; Kolli, N.; Learman, C.; Rossignol, J.; Dunbar, G.L. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5x-familial Alzheimer’s disease mice. Histochem. Cell Biol. 2016, 146, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid lipid curcumin particles provide greater anti-amyloid, anti-inflammatory and neuroprotective effects than curcumin in the 5xFAD mouse model of Alzheimer’s disease. BMC Neurosci. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Pragati, P.; Nahar, A.; Slitt, L.; Navindra, P. Anti-inflammatory effects of novel standardized solid lipid curcumin formulations. J. Med. Food 2015, 18, 786–792. [Google Scholar]
- Dadhaniya, P.; Patel, C.; Muchhara, J.; Bhadja, N.; Mathuria, N.; Vachhani, K.; Soni, M.G. Safety assessment of a solid lipid curcumin particle preparation: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2011, 49, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Sadegh-Malvajerd, S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Sharif-Zadeh, M.; Akbari-Javar, H.; Hamidi, M. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: Preparation, optimization, and pharmacokinetic evaluation. ACS Chem. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer’s disease. Colloids Surf. B Biointerfaces 2015, 134, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lazar, A.N.; Mourtas, S.; Youssef, I.; Parizot, C.; Dauphin, A.; Delatour, B.; Antimisiaris, S.G.; Duyckaerts, C. Curcumin-conjugated nanoliposomes with high affinity for Aβ deposits: Possible applications to Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Sancini, G.; Gregori, M.; Salvati, E.; Cambianica, L.; Re, F.; Ornaghi, F.; Canovi, M.; Fracasso, C.; Cagnotto, A.; Colombo, M.; et al. Functionalization with TAT-peptide enhances blood–brain barrier crossing in vitro of nanoliposomes carrying a curcumin-derivative to bind amyloid-β peptide. J. Nanomed. Nanotechnol. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Mourtas, S.; Canovi, M.; Zona, C.; Aurilia, D.; Niarakis, A.; La Ferla, B.; Salmona, M.; Nicotra, F.; Gobbi, M.; Antimisiaris, S.G. Curcumin-decorated nanoliposomes with very high affinity for amyloid beta1–42 peptide. Biomaterials 2011, 32, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, C.; Zona, C.; Sironi, E.; Colombo, L.; Messa, M.; Aurilia, D.; Gregori, M.; Masserini, M.; Salmona, M.; Nicotra, F.; et al. Curcumin derivatives as new ligands of Apeptides. J. Biotechnol. 2011, 156, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Gregori, M.; Radice, I.; Da Re, F.; Grana, D.; Re, F.; Salvati, E.; Masserini, M.; Ferrarese, C.; Zoia, C.P.; et al. Multifunctional liposomes interact with Abeta in human biological fluids: Therapeutic implications for Alzheimer’s disease. Neurochem. Int. 2017, 108, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lin, C.Y.; Li, J.S.; Lou, Y.I. Wheat germ agglutinin-conjugated liposomes incorporated with cardiolipin to improve neuronal survival in Alzheimer’s disease treatment. Int. J. Nanomed. 2017, 12, 1757–1774. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lin, C.C. Rescuing apoptotic neurons in Alzheimer’s disease using wheat germ agglutinin- conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int. J. Nanomed. 2015, 10, 2653–2672. [Google Scholar] [CrossRef] [PubMed]
- Valldeperas, M.; Wiśniewska, M.; Ram-On, M.; Kesselman, E.; Danino, D.; Nylander, T.; Barauskas, J. Sponge phases and nanoparticle dispersions in aqueous mixtures of mono- and diglycerides. Langmuir 2016, 32, 8650–8659. [Google Scholar] [CrossRef] [PubMed]
- Milak, S.; Zimmer, A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc. Chem. Res. 2011, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Štěpánek, P.; Lesieur, S. Multicompartment lipid cubic nanoparticles with high protein upload: Millisecond dynamics of formation. ACS Nano 2014, 8, 5216–5226. [Google Scholar] [CrossRef] [PubMed]
- Zerkoune, L.; Lesieur, S.; Putaux, J.L.; Choisnard, L.; Gèze, A.; Wouessidjewe, D.; Angelov, B.; Vebert-Nardin, C.; Doutch, J.; Angelova, A. Mesoporous self-assembled nanoparticles of biotransesterified cyclodextrins and nonlamellar lipids as carriers of water-insoluble substances. Soft Matter 2016, 12, 7539–7550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Shen, J.Q.; Gan, Y.; Geng, H.M.; Zhang, X.X.; Zhu, C.L.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, A.; Li, Y.; Chen, Y.; Angelova, A.; Garamus, V.M.; Li, N.; Drechsler, M.; Angelov, B.; Gong, Y. Self-assembled stable sponge type nanocarries for Brucea javanica oil delivery. Colloids Surf. B 2017, 153, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Azhari, H.; Strauss, M.; Hook, S.; Boyd, B.J.; Rizwan, S.B. Stabilising cubosomes with tween 80 as a step towards targeting lipid nanocarriers to the blood–brain barrier. Eur. J. Pharm. Biopharm. 2016, 104, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, J.; Zhang, Q.; Yan, X.; Guo, L.; Gao, X.; Qiu, M.; Jiang, X.; Lai, R.; Chen, H. A novel small odorranalectin-bearing cubosomes: Preparation, brain delivery and pharmacodynamic study on amyloid-b25–35-treated rats following intranasal administration. Eur. J. Pharm. Biopharm. 2012, 80, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Madheswaran, T.; Sundaramoorthy, P.; Kim, H.M.; Yoo, B.K. Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. Int. J. Nanomed. 2014, 9, 3119–3130. [Google Scholar] [Green Version]
- Wei, L.; Li, X.; Guo, F.; Liu, X.; Wang, Z. Structural properties, in vitro release and radical scavenging activity of lecithin based curcumin-encapsulated inverse hexagonal (HII) liquid crystals. Colloids Surf. A 2018, 539, 124–131. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural. Regen. Res. 2017, 12, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid crystalline nanostructures as pegylated reservoirs of omega-3 polyunsaturated fatty acids: Structural insights toward delivery formulations against neurodegenerative disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Guerzoni, L.P.; Nicolas, V.; Angelova, A. In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharm. Res. 2017, 34, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Angelova, A.; Angelov, B.; Drechsler, M.; Garamus, V.M.; Willumeit-Römere, R.; Zou, A. Sterically stabilized spongosomes for multi-drug delivery of anticancer nanomedicines. J. Mater. Chem. B 2015, 3, 7734–7744. [Google Scholar] [CrossRef]
- Biswas, A.; Kurkute, P.; Jana, B.; Laskar, A.; Ghosh, S. An amyloid inhibitor octapeptide forms amyloid type fibrous aggregates and affects microtubule motility. Chem. Commun. 2014, 50, 2604–2607. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Das, G.; Barman, S.; Mohapatra, S.; Bhunia, D.; Jana, B.; Ghosh, S. Biodegradable neuro-compatible peptide hydrogel promotes neurite outgrowth, shows significant neuroprotection, and delivers anti-Alzheimer drug. ACS Appl. Mater. Interfaces 2017, 9, 5067–5076. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-based drug delivery systems. J. Pharm. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ganem-Quintanar, A.; Quintanar-Guerrero, D.; Buri, P. Monoolein: A review of the pharmaceutical applications. Drug Dev. Ind. Pharm. 2000, 26, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N.; Cicchetti, F.; Calon, F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J. 2008, 22, 1213–1225. [Google Scholar] [CrossRef] [PubMed]



| Diseases | Characteristics | Genetics factors | Symptoms | Actual treatments |
|---|---|---|---|---|
| AD | Senile plaques from extracellular amyloid-Aβ accumulation, Intracellular neurofibrillary tangles, Tau protein aggregation, Irreversible neuronal loss, Brain atrophy | Inherited form (70% of patients): mutations of APP, PSEN1 or PSEN2. Sporadic form (30%): presence of ApoE4 allele in the ApoE gene | Progressive memory loss, Decision judgement loss, Autonomy loss | Anticholinergics (tacrine, rivastigmine, galantamine and donepezil), Memantine, Antipsychotics, NSAIDs |
| PD | α-Synucleinopathy, Presence of Lewy bodies, Degeneration of dopaminergic neurons in the substance nigra of the brain, Dopamine deficiency | Gene mutations: α-synuclein SNCA, Parkin PRKN, PARK7, PINK1, LRRK2, GBA, DJ-1, VPS35, EIF4G1, DNAJC13 and CHCHD2 | Hypokinesia, Bradykinesia, Rigidity, Postural instability, Neuropsychiatric disturbances | Levodopa, Dopamine agonists, MAO-B inhibitors, COMT inhibitors, Anticholinergics |
| HD | Accumulation of mutant Huntingtin protein in the brain | Expansion of CAG trinucleotide in Huntingtin gene (HTT) | Chorea, Cognitive and neuropsychiatric disorders | Tetrabenazine, Neuroleptics, Antipsychotics |
| ALS | Progressive degeneration of motor neurons | Sporadic form: 90% of patients Inherited form: 10% Mutations of SOD1, TARDBP, FUS, UBQLN2, OPTN, and C9ORF72 genes | Spasms, Muscle atrophy, Squelettal muscle paralysis, Cognitive or behavioral dysfunction | Riluzole |
| Disease | Model/Administration Route | Mechanism | Outcomes |
|---|---|---|---|
| AD | In vitro: human neuroblastoma SH-SY5Y and IMR-32 cells | Enhancement of the expression of DNA repair enzymes (APE1, pol β, and PARP1 1) to halt the oxidative DNA base damage via base excision repair (BER) pathway; Activation of the antioxidant response element (ARE) via Nrf2 upregulation | Revitalization of the neuronal cells from Aβ 2 induced oxidative stress [41]. |
| AD | In vitro: mouse hippocampal clone neuronal cell line HT-22 cells treated with Aβ 1-42, In vivo: mice with APP/PS1 transgenes | Decrease of the autophagosomes number, Increase of the lysosomal Ca2+ regulation of PI(3,5)P2 and Transient Receptor Potential Mucolipin-1 Expression (TRPME) | Neuronal cell growth, Protective role of CU on memory and cognition impairments [42]. |
| AD | In vivo: rat, oral supplementation | Increase of GPx 3, CAT 4, GSH 5 activities and Ach 6 levels | Improving memory and cognitive abilities [43]. |
| PD | In vivo: Drosophila model of PD with dUCH 7 knockdown | Effects on dUCH 7 knockdown, a homolog of human UCH-L1 | Decrease of ROS levels, Improved locomotive abilities, Reduction of dopaminergic neurons degeneration [44]. |
| PD | In vivo: male Sprague-Dawley rats injured by 6-OHDA 8 in the left striatum | Activation of the Wnt/β-catenin signaling pathway, Higher Wnt3a and β-catenin mRNA and protein expressions, c-myc and cyclin D1 mRNA expression, enhanced SOD 9 and GPx 3 contents, decreased MDA 10 content and elevated mitochondrial membrane potential | Protective effect of CU against oxidative stress-induced injury, Enhanced viability, survival, and adhesion, attenuated apoptosis of deutocerebrum primary cells [45]. |
| PD | In vivo: MPTP 11 mice, intranasal mode of administration of CU (mucoadhesive system) | Increase of dopamine concentration in brain, which improves muscular coordination and gross behavioral activities of the test animal, Significant reduction of the MPTP11-mediated dopamine depletion | Improvement in motor performance, Symptomatic neuroprotection against MPTP-induced neurodegeneration in the striatum [46]. |
| HD | In vivo: CAG140 mice, a knock-in (KI) mouse model of HD | Decreased huntingtin aggregates, increased striatal DARPP-32 and D1 receptor mRNAs | Partial improvement of transcriptional deficits, partial behavioral improvement [47]. |
| Diazepam-induced cognitive impairment | In vivo: diazepam-treated rats, oral supplementation | Downregulation of the extracellular signal-regulated kinase (ERK 1/2)/nuclear transcription factor-(NF-)κB/pNF-κB pathway in the hippocampus and the iNOS 12 expression in the hippocampus and frontal cortex | Improvement of the cognitive performance, Decrease of blood and brain oxidative stress levels [48]. |
| Alcohol-induced neurodege neration | In vivo: rat, oral supplementation | Decrease of the reduced form of GSH 5, SOD 9, GPx 3, GR 13, change in the Bcl-2 levels, Activation of the CREB-BDNF signaling pathway | Neuroprotection against alcohol-induced oxidative stress, apoptosis and inflammation [49]. |
| Nicotine-induced neurodege neration | In vivo: rat, oral supplementation | Activation of the CREB-BDNF signaling pathway | Neuroprotection against nicotine-induced inflammation, apoptosis and oxidative stress, Reduction of the motor activity disturbances [50]. |
| Disease | Nanoformulation Type | Model/Administration Route | Outcomes |
|---|---|---|---|
| AD | PLGA 1 nanoparticles | In vitro: SK-N-SH human neuroblastoma cells | Protection against H2O2-induced oxidative damage [70]. |
| AD | PLGA nanoparticles | In vitro: Neural stem cells, In vivo: Aβ 2-amyloid induced rat model of AD-like phenotypes | Expression of genes involved in neuronal proliferation and differentiation, Reverse learning and memory impairments [73]. |
| AD | PLGA nanoparticles conjugated with Tet-1 peptide | In vitro | Anti-amyloid activity unchanged, decrease of aggregates size [74], Diminution of anti-oxidant activity. |
| AD | PLGA nanoparticles functionalized with glutathione | In vitro: in SK-N-SH cells | Neuronal uptake, Enhanced curcumin action [75,76]. |
| AD | PLGA nanoparticles | In vivo: Rat, IV, oral | Increased CU bioavailability and plasma concentration [77]. |
| AD | PLGA nanoparticles | In vivo: Rat | Prolonged CU retention time in cerebral cortex and hippocampus [78]. |
| AD | Apolipoprotein E3-mediated poly(butyl)cyano acrylate nanoparticles | In vitro: SH-SY5Y cells | Protection against Aβ-induced cytotoxicity [79]. |
| AD | Pegylated poly(alkyl cyanoacrylate) nanoparticles with anti-Aβ 1–42 antibody at the surface | In vitro | Inhibition of Aβ aggregation [80]. |
| AD | Spherical (SPNs) or Discoidal (DPNs) polymeric nanocontructs PLGA, DSPE-PEG 3 | In vitro: Raw 264.7 cells In vitro production of Aβ fibers | Decrease of the pro-inflammatory cytokines in macrophages stimulated via Aβ fibers [81] |
| AD | Polymeric nanoparticles (NanoCurcTM) | In vitro: SK-N-SH differentiated cells In vivo: Mice, parenteral injection | Protection against H2O2-induced oxidative stress, Downregulation of caspase 3 and 7 activities, mediators of the apoptotic pathway, Increased glutathione levels [82]. |
| AD | Nanocurcumin CU within polyethylene glycol-polylactide diblock polymer micelles | In vitro In vivo: AD model Tg2576 mice | Higher curcumin concentration in plasma, 6 times higher area under the curve and mean residence time in brain than ordinary CU, Improved memory function [83]. |
| AD | Nanoemulsion | In vitro: SK-N-SH cell line, Sheep nasal mucosa | Safe for intranasal delivery for brain targeting, Higher flux and permeation across sheep nasal mucosa [84]. |
| Mitochon drial dysfunction in brain aging | Micelles | In vitro: PC12 cells In vivo: NMRI mice; Ex vivo: isolated mouse brain mitochondria | Improved bioavailability of native curcumin around 10- to 40-fold in plasma and brain of mice, Prevention of mitochondrial swelling in isolated mouse brain mitochondria, Protection of PC12 cells from nitrosative stress as compared to free CU [85]. |
| PD | Alginate nanocomposites | In vivo: Drosophila, oral | Reduction of oxidative stress and apoptosis in the brain [86]. |
| Disease | Nanoformulation Type | Model/Administration Route | Outcomes |
|---|---|---|---|
| AD | Solid lipid nanoparticles | In vitro: Mouse neuroblastoma cells after Aβ 1 exposure | Decreased ROS production, Prevented apoptotic death, Inhibition of Tau formation [89,90]. |
| AD | Solid lipid curcumin particle (SLCP), Longvida® | In vitro: lipopolysaccharide (LPS)-stimulated RAW 264.7 cultured murine macrophages. | Improved solubility over unformulated curcumin, Decreased LPS induced pro-inflammatory mediators NO, PGE2, and IL-6 by inhibiting the activation of NF-kB [92]. |
| AD | Solid lipid particleswith CU (SLCP) | In vivo: one-year-old 5xFAD-and age-matched wild-type mice, intraperitoneal injections of CU/SLCP | Decrease in Aβ plaque loads in dentate gyrus of hippocampus, More anti-amyloid, anti-inflammatory, and neuroprotective [91]. |
| AD | Solid lipid nanoparticles | In vivo: Rat, oral | Effective delivery across the BBB 2 [88]. |
| HD | Solid lipid nanoparticles (CU-SLNs) | In vivo: (3-NP)-induced HD in rats | Restored glutathione levels and superoxide dismutase activity, Activation of nuclear factor-erythroid 2 antioxidant pathway, Reduction in mitochondrial swelling, lipid peroxidation, protein carbonyls and reactive oxygen species [89]. |
| CNS disorders | Solid lipid nanoparticles (CU-SLNs) and nanostructured lipid carriers (CU-NLCs) | In vivo: male Sprague−Dawley rats 6−8 weeks old, oral | Enhanced curcumin brain uptake, Cur-NLCs enhance the absorption of brain curcumin more than 4-folds in comparison with Cur-SLNs [95]. |
| AD | Lipoprotein (LDL)-mimic nanostructured lipid carrier (NLC) modified with lactoferrin (Lf) and loaded with CU | In vivo: Rat, oral | Cellular uptake mediated by the Lf receptor, Permeability through the BBB and preferentially accumulation in the brain, Efficacy in controlling the damage associated with AD [96]. |
| AD | Liposomes functionalized with TAT-peptide | In vitro | Permeability across the BBB enhanced [98]. |
| AD | Liposomes containing cardiolipin | In vitro: SK-N-MC cells | Inhibition of the phosphorylation of p38, JNK, and tau protein, Protection against serious degeneration of Aβ insulted neurons [101]. |
| AD | WGA 3-conjugated and cardiolipin-incorporated liposomes carrying NGF 4 and CU | In vitro: Human astrocytes and to protect SK-N-MC cells Apoptosis induced by β-amyloid1–42 (Aβ 1–42) fibrils | Increased entrapment efficiency of NGF and CU, of NGF release and cell viability, Decreased release of CU, Permeability of NGF and CU across the blood–brain barrier [102]. |
| AD | Liposomes | In vivo: Mice, stereotaxic injection in the right hippocampus and neocortex | Decrease in Aβ secretion and toxicity [97]. |
| AD | Liposomes decorated with anti-transferrin receptor mAb | In vivo injection, hippocampus and neocortex | Decrease in Aβ 1–42 aggregation, Internalization in the BBB model [99]. |
| AD | Liposomes functionalized with a curcumin-alkyne derivative TREG | Human biological fluids from sporadic AD patients and down syndrome subjects | Sequestration of Aβ 1–42 [100,101]. |
| Neuronal loss | Liquid-crystalline lipid nanoparticles carrying curcumin and DHA | In vitro: SH-SY5Y cells | Neuronal viability and neurite outgrowth by activation of the TrkB receptor signaling, and promotion of phosphorylated CREB protein expression [118]. |
| AD | Lipopeptide: a short microtubule- stabilizing peptide conjugated to a hydrophobic palmitic acid chain | In vitro: Neuro-2a cells, PC-12 differentiated cells | Neurite outgrowth in absence of external growth factors, Neural cells morphology and health amelioration [120,121]. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakotoarisoa, M.; Angelova, A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines 2018, 5, 126. https://doi.org/10.3390/medicines5040126
Rakotoarisoa M, Angelova A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines. 2018; 5(4):126. https://doi.org/10.3390/medicines5040126
Chicago/Turabian StyleRakotoarisoa, Miora, and Angelina Angelova. 2018. "Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders" Medicines 5, no. 4: 126. https://doi.org/10.3390/medicines5040126
APA StyleRakotoarisoa, M., & Angelova, A. (2018). Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines, 5(4), 126. https://doi.org/10.3390/medicines5040126