Screening of Crude Drugs Used in Japanese Kampo Formulas for Autophagy-Mediated Cell Survival of the Human Hepatocellular Carcinoma Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Crude Drug Extracts
2.2. Reagents
2.3. Cell Culture and Treatment
2.4. Western Blot Analysis
2.5. Determination of Cell Viability
2.6. Statistical Analyses
3. Results
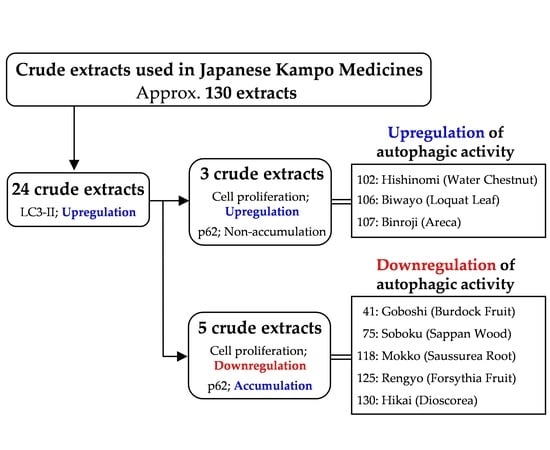
3.1. Screening the Crude Drugs for LC3-II Expression
3.2. Effects of the Selected 24 Crude Drugs on Cell Viability
3.3. Effects of Eight Crude Drugs on P62 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef]
- Augustine, M.K.; Choi, M.D.; Stefan, W.; Ryter, P.H.D.; Beth Levine, M.D. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Lassen, K.G.; Xavier, R.J. Mechanisms and function of autophagy in intestinal disease. Autophagy 2018, 14, 216–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, T.; Komatsu, M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Kroemer, G. Autophagy in stress and disease. Cell Death Differ. 2015, 22, 365–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Yamamoto, A.; Oshitani-Okamoto, S.; Ohsumi, Y.; Yoshimori, T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 2004, 117, 2805–2812. [Google Scholar] [CrossRef] [Green Version]
- Geetha, T.; Wooten, M.W. Structure and functional properties of the ubiquitin binding protein p62. FEBS Lett. 2002, 512, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Seibenhener, M.L.; Babu, J.R.; Geetha, T.; Wong, H.C.; Krishna, N.R.; Wooten, M.W. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol. Cell. Biol. 2004, 24, 8055–8068. [Google Scholar] [CrossRef]
- Johansen, T.; Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011, 7, 279–296. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Mizushima, N. p62 Targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J. Cell. Biol. 2011, 192, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcotte, S.; Chan, D.A.; Sutphin, P.D.; Hay, M.P.; Denny, W.A.; Giaccia, A.J. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell 2008, 14, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Marinković, M.; Šprung, M.; Buljubašić, M.; Novak, I. Autophagy modulation in cancer: Current knowledge on action and therapy. Oxid. Med. Cell. Longev. 2018, 2018, 8023821. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, Z.Q.; Wang, B.; Jiang, H.X.; Cheng, L.; Shen, L.M. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014, 14, 49. [Google Scholar] [CrossRef]
- La, X.; Zhang, L.; Li, Z.; Yang, P.; Wang, Y. Berberine-induced autophagic cell death by elevating GRP78 levels in cancer cells. Oncotarget 2017, 8, 20909–20924. [Google Scholar] [CrossRef] [Green Version]
- Nozaki, R.; Kono, T.; Bochimoto, H.; Watanabe, T.; Oketani, K.; Sakamaki, Y.; Okubo, N.; Nakagawa, K.; Takeda, H. Zanthoxylum fruit extract from Japanese pepper promotes autophagic cell death in cancer cells. Oncotarget 2016, 7, 70437–70446. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Wang, Z.; Wang, Y.; Guo, D.; Yang, J.; Chen, L.; Tan, N. Natural cyclopeptide RA-XII, a new autophagy inhibitor, suppresses protective autophagy for enhancing apoptosis through AMPK/mTOR/P70S6K pathways in HepG2 cells. Molecules 2017, 22, 1934. [Google Scholar] [CrossRef] [PubMed]
- Young, A.N.; Herrera, D.; Huntsman, A.C.; Korkmaz, M.A.; Lantvit, D.D.; Mazumder, S.; Kolli, S.; Coss, C.C.; King, S.; Wang, H.; et al. Phyllanthusmin derivatives induce apoptosis and reduce tumor burden in high-grade serous ovarian cancer by late-stage autophagy inhibition. Mol. Cancer Ther. 2018, 17, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Toyama, T.; Sato, T.; Suzuki, M.; Morozumi, A.; Sakagami, H.; Hamada, N. Kampo therapies and the use of herbal medicines in the dentistry in Japan. Medicines 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sekiguchi, K.; Kawakami, Z.; Watanabe, J.; Mizoguchi, K.; Ikarashi, Y.; Yamamoto, M. Basic study of drug-drug interaction between memantine and the traditional Japanese Kampo medicine yokukansan. Molecules 2018, 24, 115. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Watanabe, T.; Hoshino, T.; Suda, N.; Mori, K.; Yasui, T.; Yamauchi, N.; Kashiwagi, H.; Gomi, T.; Oizumi, T.; et al. Recent progress of basic studies of natural products and their dental application. Medicines 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, M.; Yamaguchi, K.; Tsukada, M.; Ebihara, N.; Ikemoto, H.; Hisamitsu, T. Kampo (traditional Japanese herbal) formulae for treatment of stomatitis and oral mucositis. Medicines 2018, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Amitani, M.; Amitani, H.; Sloan, R.A.; Suzuki, H.; Sameshima, N.; Asakawa, A.; Nerome, Y.; Owaki, T.; Inui, A.; Hoshino, E. The translational aspect of complementary and alternative medicine for cancer with particular emphasis on Kampo. Front. Pharmacol. 2015, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Li, W.; Takemoto, H.; Takeuchi, M.; Nakamura, A.; Tokura, E.; Akahane, C.; Ueno, K.; Komatsu, K.; Kuriyama, N.; et al. Comprehensive evaluation ofantioxidant effects of Japanese Kampo medicines led to identification of Tsudosan formulation as a potent antioxidant agent. J. Nat. Med. 2019, 1, 163–172. [Google Scholar] [CrossRef]
- Kobayashi, Y. Kampo medicine in the new model core curriculum of pharmaceutical education. Yakugaku Zasshi 2016, 136, 423–432. [Google Scholar] [CrossRef]
- Uto, T.; Morinaga, O.; Tanaka, H.; Shoyama, Y. Analysis of the synergistic effect of glycyrrhizin and other constituents in licorice extract on lipopolysaccharide-induced nitric oxide production using knock-out extract. Biochem. Biophys. Res. Commun. 2012, 417, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Tung, N.H.; Taniyama, R.; Miyanowaki, T.; Morinaga, O.; Shoyama, Y. Anti-inflammatory activity of constituents isolated from aerial part of Angelica acutiloba Kitagawa. Phytother. Res. 2015, 12, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Okubo, S.; Uto, T.; Goto, A.; Tanaka, H.; Nishioku, T.; Yamada, K.; Shoyama, Y. Berberine induces apoptotic cell death via activation of caspase-3 and -8 in HL-60 human leukemia cells: Nuclear localization and structure-activity relationships. Am. J. Chin. Med. 2017, 45, 1497–1511. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Tung, N.H.; Ohta, T.; Juengsanguanpornsuk, W.; Hung, L.Q.; Hai, N.T.; Long, D.D.; Thuong, P.T.; Okubo, S.; Hirata, S.; et al. Antiproliferative activity and apoptosis induction by trijuganone C isolated from the root of Salvia miltiorrhiza Bunge (Danshen). Phytother. Res. 2018, 32, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Mayurbhai, H.S.; Itakura, E.; Mizushima, N. Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 2014, 10, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Gravitz, L. Liver cancer. Nature 2014, 516, 7529. [Google Scholar] [CrossRef]
- Takamura, A.; Komatsu, M.; Hara, T.; Sakamoto, A.; Kishi, C.; Waguri, S.; Eishi, Y.; Hino, O.; Tanaka, K.; Mizushima, N. Autophagy-deficient mice develop multiple liver tumors. Genes. Dev. 2011, 25, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Waguri, S.; Ueno, T.; Iwata, J.; Murata, S.; Tanida, I.; Ezaki, J.; Mizushima, N.; Ohsumi, Y.; Uchiyama, Y.; et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005, 169, 425–434. [Google Scholar] [CrossRef]
- Wu, D.H.; Jia, C.C.; Chen, J.; Lin, Z.X.; Ruan, D.Y.; Li, X.; Lin, Q.; Min-Dong; Ma, X.K.; Wan, X.B.; Cheng, N.; et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumour Biol. 2014, 12225–12233. [Google Scholar] [CrossRef]
- Yen, C.Y.; Chiang, W.F.; Liu, S.Y.; Lin, C.C.; Liao, K.A.; Lin, C.Y.; Hsieh, W.F.; Cheng, Y.C.; Hsu, KC.; Lin, P.Y.; Chen, T.C.; et al. Impacts of autophagy-inducing ingredient of areca nut on tumor cells. PLoS ONE 2015, 10, e0128011. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, C.M.; Shao, Z.; Zhao, X.P.; Wang, M.; Yan, T.L.; Zhou, X.C.; Jiang, E.H.; Liu, K.; Shang, Z.J. Autophagy induced by areca nut extract contributes to decreasing cisplatin toxicity in oral squamous cell carcinoma cells: Roles of reactive oxygen species/AMPK signaling. Int. J. Mol. Sci. 2017, 18, 524. [Google Scholar] [CrossRef]
- Feng, Q.; Yao, J.; Zhou, G.; Xia, W.; Lyu, J.; Li, X.; Zhao, T.; Zhang, G.; Zhao, N.; Yang, J. Quantitative proteomic analysis reveals that arctigenin alleviates Concanavalin A-induced hepatitis through suppressing immune system and regulating autophagy. Front. Immunol. 2018, 9, 1881. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; He, P.; Wang, H.; Ye, Y.; Li, X.; Xie, P.; Wu, B. Brazilin induces FOXO3A-dependent autophagic cell death by disturbing calcium homeostasis in osteosarcoma cells. Cancer Chemother. Pharmacol. 2018, 82, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, J.; Wu, Y.; Gui, J.; Shen, Y. Costunolide inhibits the growth of OAW42-A multidrug-resistant human ovarian cancer cells by activating apoptotic and autophagic pathways, production of reactive oxygen species (ROS), cleaved caspase-3 and cleaved caspase-9. Med. Sci. Monit. 2019, 25, 3231–3237. [Google Scholar] [CrossRef] [PubMed]



| Drug No. | Japanese Name | English Name | Concentration (µg/mL) | ||
|---|---|---|---|---|---|
| 5 | 10 | 20 | |||
| 5 | Uzu | Aconite Root | 100.4 | 100.3 | 103.3 |
| 12 | Onji | Polygala Root | 100.4 | 97.3 | 96.4 |
| 17 | Kakko | Pogostemon Herb | 96.6 | 100.2 | 100.5 |
| 18 | Kakkon | Pueraria Root | 101.6 | 107.4 | 109.5 |
| 24 | Kikyo | Platycodon Root | 103.6 | 106.9 | 106.8 |
| 41 (b) | Goboshi | Burdock Fruit | 89.5 | 82.9 | 76.7 |
| 42 | Gomishi | Schisandra Fruit | 105.5 | 107.3 | 106.2 |
| 43 | Saiko | Bupleurum Root | 100.8 | 100.3 | 96.1 |
| 61 | Jashoshi | Cnidium Monnieri Fruit | 96.1 | 96.1 | 92.6 |
| 68 | Shoma | Cimicifuga Rhizome | 100.2 | 104.3 | 106.3 |
| 72 | Sentai | Cicada Slough | 105.6 | 103.2 | 101.4 |
| 75 (b) | Soboku | Sappan Wood | 100.8 | 94.0 | 86.9 |
| 76 | Soyo | Perilla Herb | 102.7 | 103.8 | 105.9 |
| 82 | Chimo | Anemarrhena Rhizome | 99.4 | 100.8 | 96.9 |
| 102 (a) | Hishinomi | Water Chestnut | 105.1 | 108.1 | 116.3 |
| 106 (a) | Biwayo | Loquat Leaf | 106.9 | 110.7 | 113.9 |
| 107 (a) | Binroji | Areca | 105.1 | 114.6 | 108.7 |
| 114 | Mao | Ephedra Herb | 101.5 | 95.0 | 97.7 |
| 118 (b) | Mokko | Saussurea Root | 94.2 | 82.3 | 67.3 |
| 124 | Ryokyo | Alpinia Officinarum Rhizome | 101.4 | 103.2 | 109.5 |
| 125 (b) | Rengyo | Forsythia Fruit | 90.4 | 89.5 | 88.7 |
| 126 | Renniku | Nelumbo Seed | 103.5 | 105.6 | 103.2 |
| 127 | Tanjin | Salvia Miltiorrhiza Root | 98.2 | 99.8 | 95.3 |
| 130 (b) | Hikai | Dioscorea | 71.8 | 50.1 | 20.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okubo, S.; Komori, H.; Kuwahara, A.; Ohta, T.; Shoyama, Y.; Uto, T. Screening of Crude Drugs Used in Japanese Kampo Formulas for Autophagy-Mediated Cell Survival of the Human Hepatocellular Carcinoma Cell Line. Medicines 2019, 6, 63. https://doi.org/10.3390/medicines6020063
Okubo S, Komori H, Kuwahara A, Ohta T, Shoyama Y, Uto T. Screening of Crude Drugs Used in Japanese Kampo Formulas for Autophagy-Mediated Cell Survival of the Human Hepatocellular Carcinoma Cell Line. Medicines. 2019; 6(2):63. https://doi.org/10.3390/medicines6020063
Chicago/Turabian StyleOkubo, Shinya, Hisa Komori, Asuka Kuwahara, Tomoe Ohta, Yukihiro Shoyama, and Takuhiro Uto. 2019. "Screening of Crude Drugs Used in Japanese Kampo Formulas for Autophagy-Mediated Cell Survival of the Human Hepatocellular Carcinoma Cell Line" Medicines 6, no. 2: 63. https://doi.org/10.3390/medicines6020063
APA StyleOkubo, S., Komori, H., Kuwahara, A., Ohta, T., Shoyama, Y., & Uto, T. (2019). Screening of Crude Drugs Used in Japanese Kampo Formulas for Autophagy-Mediated Cell Survival of the Human Hepatocellular Carcinoma Cell Line. Medicines, 6(2), 63. https://doi.org/10.3390/medicines6020063