The Effect of Tissue Stromal Vascular Fraction as Compared to Cellular Stromal Vascular Fraction to Treat Anal Sphincter Incontinence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of cSVF and tSVF
DIL Labeling of cSVF and tSVF
2.2. Animal Model
2.3. Functional Assay
2.4. Masson Trichrome Stain
2.5. Immunofluorescence Staining
2.6. Data Analysis
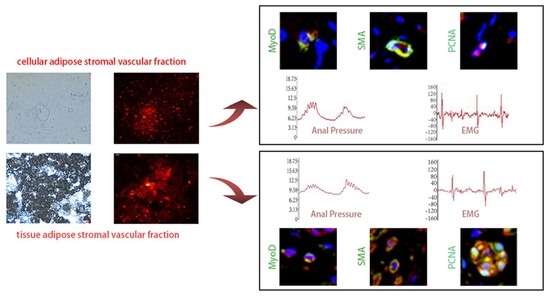
3. Results
3.1. Extraction and DIL Labeling of cSVF and tSVF
3.2. Anal Pressure Results
3.2.1. Resting Anal Pressure Values
3.2.2. Peak Anal Pressure
3.3. Electromyography Test Results
3.3.1. Electromyographic Frequencies
3.3.2. EMG Amplitude
3.4. Surface Healing of the Injury Site
3.5. Masson Trichrome Stain
3.6. Immunofluorescence Staining of cSVF and tSVF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatoor, D.R.; Taylor, S.J.; Cohen, C.R.; Emmanuel, A.V. Faecal incontinence. Br. J. Surg. 2007, 94, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Meyer, I.; Richter, H.E. Impact of fecal incontinence and its treatment on quality of life in women. Women’s Health 2015, 11, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C. Pathophysiology of adult fecal incontinence. Gastroenterology 2004, 126, S14–S22. [Google Scholar] [CrossRef] [PubMed]
- LaCross, A.; Groff, M.; Smaldone, A. Obstetric Anal Sphincter Injury and Anal Incontinence Following Vaginal Birth: A Systematic Review and Meta-Analysis. J. Midwifery Women’s Health 2015, 60, 37–47. [Google Scholar] [CrossRef] [PubMed]
- De Ligny, W.R.; Kerkhof, M.H.; Ruiz-Zapata, A.M. Regenerative medicine as a therapeutic option for fecal incontinence: A systematic review of preclinical and clinical studies. Am. J. Obstet. Gynecol. 2019, 220, 142–154. [Google Scholar] [CrossRef]
- Balaphas, A.; Meyer, J.; Meier, R.; Liot, E.; Buchs, N.C.; Roche, B.; Toso, C.; Buhler, L.H.; Gonelle-Gispert, C.; Ris, F. Cell Therapy for Anal Sphincter Incontinence: Where Do We Stand? Cells 2021, 10, 2086. [Google Scholar] [CrossRef]
- Zorcolo, L.; Covotta, L.; Bartolo, D.C. Outcome of anterior sphincter repair for obstetric injury: Comparison of early and late results. Dis. Colon Rectum 2005, 48, 524–531. [Google Scholar] [CrossRef]
- Gronewold, M.; Kroencke, T.; Hagedorn, A.; Tunn, R.; Gauruder-Burmester, A. External anal sphincter repair using the overlapping technique in patients with anal incontinence and concomitant pudendal nerve damage. Zent. Chir. 2008, 133, 129–134. [Google Scholar] [CrossRef]
- Demirbas, S.; Atay, V.; Sucullu, I.; Filiz, A.I. Overlapping Repair in Patients with Anal Sphincter Injury. Med. Princ. Pract. 2007, 17, 56–60. [Google Scholar] [CrossRef]
- Brown, S.R.; Wadhawan, H.; Nelson, R.L. Surgery for faecal incontinence in adults. Cochrane Database Syst. Rev. 2013, 2013, D1757. [Google Scholar] [CrossRef]
- Deng, Z.; Jin, J.; Wang, S.; Qi, F.; Chen, X.; Liu, C.; Li, Y.; Ma, Y.; Lyu, F.; Zheng, Q. Narrative review of the choices of stem cell sources and hydrogels for cartilage tissue engineering. Ann. Transl. Med. 2020, 8, 1598. [Google Scholar] [CrossRef]
- Qi, F.; Deng, Z.; Ma, Y.; Wang, S.; Liu, C.; Lyu, F.; Wang, T.; Zheng, Q. From the perspective of embryonic tendon development: Various cells applied to tendon tissue engineering. Ann. Transl. Med. 2020, 8, 131. [Google Scholar] [CrossRef]
- Leung, V.Y.; Aladin, D.M.; Lv, F.; Tam, V.; Sun, Y.; Lau, R.Y.; Hung, S.C.; Ngan, A.H.; Tang, B.; Lim, C.T.; et al. Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells 2014, 32, 2164–2177. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Lyu, F.J.; Gao, J.; Lin, J.; Liu, J.; Chen, X.; Li, Z.; Shan, J.; Wu, J. The therapeutic effect of stem cells from human exfoliated deciduous teeth on a rat model of tracheal fistula. Stem Cell Res. Ther. 2022, 13, 310. [Google Scholar] [CrossRef]
- Drommer, R.B.; Mende, U.; Krifka, F.J. Free fatty tissue transplantation in the area of the face. Hautarzt 1995, 46, 628–631. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Tanikawa, D.; Aguena, M.; Bueno, D.F.; Passos-Bueno, M.R.; Alonso, N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast. Reconstr. Surg. 2013, 132, 141–152. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Paspaliaris, V.; Koliakos, G.; Ntolios, P.; Bouros, E.; Oikonomou, A.; Zissimopoulos, A.; Boussios, N.; Dardzinski, B.; Gritzalis, D.; et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Sanz-Ruiz, R.; Sanchez, P.L.; Lasso, J.; Perez-Cano, R.; Alonso-Farto, J.C.; Perez-David, E.; Fernandez-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014, 168, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Trivisonno, A.; Alexander, R.W.; Baldari, S.; Cohen, S.R.; Di Rocco, G.; Gentile, P.; Magalon, G.; Magalon, J.; Miller, R.B.; Womack, H.; et al. Intraoperative Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell- and Tissue-Based Therapies: Concise Review. Stem Cells Transl. Med. 2019, 8, 1265–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eto, H.; Suga, H.; Matsumoto, D.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Yoshimura, K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast. Reconstr. Surg. 2009, 124, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- van Dongen, J.A.; Stevens, H.P.; Parvizi, M.; van der Lei, B.; Harmsen, M.C. The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair. Regen. 2016, 24, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- van Boxtel, J.; Vonk, L.A.; Stevens, H.P.; van Dongen, J.A. Mechanically Derived Tissue Stromal Vascular Fraction Acts Anti-inflammatory on TNF Alpha-Stimulated Chondrocytes In Vitro. Bioengineering 2022, 9, 345. [Google Scholar] [CrossRef]
- Bluguermann, C.; Wu, L.; Petrigliano, F.; McAllister, D.; Miriuka, S.; Evseenko, D.A. Novel aspects of parenchymal-mesenchymal interactions: From cell types to molecules and beyond. Cell Biochem. Funct. 2013, 31, 271–280. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesthet. Plast. Surg. 2020, 44, 1258–1265. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galie, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Carstens, M.H.; Quintana, F.J.; Calderwood, S.T.; Sevilla, J.P.; Rios, A.B.; Rivera, C.M.; Calero, D.W.; Zelaya, M.L.; Garcia, N.; Bertram, K.A.; et al. Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: Safety and evidence of efficacy at 1 year. Stem Cells Transl. Med. 2021, 10, 1138–1147. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, T.; Zhao, F.; Song, K.; Xu, L.; Xu, Z.; Zhou, C.; Qin, Z.; Xu, Z.; Wu, R.; et al. Effect of uncultured adipose-derived stromal vascular fraction on preventing urethral stricture formation in rats. Sci. Rep. 2022, 12, 3573. [Google Scholar] [CrossRef]
- Guillo, L.; Grimaud, F.; Houser, F.; Prost, C.; Jouve, E.; Philandrianos, C.; Abellan, M.; Veran, J.; Visee, C.; Beyer-Berjot, L.; et al. Three-year outcome of local injection of autologous stromal vascular fraction cells and microfat in refractory perianal fistulas of Crohn’s disease. Stem Cell Res. Ther. 2022, 13, 67. [Google Scholar] [CrossRef]
- Ghiasloo, M.; Lobato, R.C.; Diaz, J.M.; Singh, K.; Verpaele, A.; Tonnard, P. Expanding Clinical Indications of Mechanically Isolated Stromal Vascular Fraction: A Systematic Review. Aesthet. Surg. J. 2020, 40, P546–P560. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Tuin, A.J.; Spiekman, M.; Jansma, J.; van der Lei, B.; Harmsen, M.C. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: A systematic review. J. Tissue Eng. Regen. Med. 2018, 12, e261–e274. [Google Scholar] [CrossRef] [Green Version]
- Gimble, J.M.; Guilak, F.; Bunnell, B.A. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res. Ther. 2010, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Hakakian, C.S. A novel and effective strategy for the isolation of adipose-derived stem cells: Minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast. Reconstr. Surg. 2015, 135, 454e. [Google Scholar] [CrossRef]
- Salcedo, L.; Penn, M.; Damaser, M.; Balog, B.; Zutshi, M. Functional Outcome After Anal Sphincter Injury and Treatment With Mesenchymal Stem Cells. Stem Cells Transl. Med. 2014, 3, 760–767. [Google Scholar] [CrossRef]
- Chen, W.; He, Z.; Li, S.; Wu, Z.; Tan, J.; Yang, W.; Li, G.; Pan, X.; Liu, Y.; Lyu, F.; et al. The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats. Bioengineering 2022, 9, 318. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, Y.C.; Wilkison, W.O.; Gimble, J.M. Adipose-derived stromal cells--Their utility and potential in bone formation. Int. J. Obes. Relat. Metab. Disord. 2000, 24 (Suppl. 4), S41–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; Andre, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Rangappa, S.; Fen, C.; Lee, E.H.; Bongso, A.; Sim, E.K. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann. Thorac. Surg. 2003, 75, 775–779. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Banks, V.; Peluso, D.P.; Morris, E.A. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J. Cell Physiol. 2000, 185, 98–106. [Google Scholar] [CrossRef]
- Konno, M.; Hamazaki, T.S.; Fukuda, S.; Tokuhara, M.; Uchiyama, H.; Okazawa, H.; Okochi, H.; Asashima, M. Efficiently differentiating vascular endothelial cells from adipose tissue-derived mesenchymal stem cells in serum-free culture. Biochem. Biophys. Res. Commun. 2010, 400, 461–465. [Google Scholar] [CrossRef]
- Xu, Y.; Malladi, P.; Wagner, D.R.; Longaker, M.T. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr. Opin. Mol. Ther. 2005, 7, 300. [Google Scholar]
- Kapur, S.K.; Katz, A.J. Review of the adipose derived stem cell secretome. Biochimie 2013, 95, 2222–2228. [Google Scholar] [CrossRef]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Huang, S.; Leung, V.; Peng, S.; Li, L.; Lu, F.J.; Wang, T.; Lu, W.; Cheung, K.M.C.; Zhou, G. Developmental definition of MSCs: New insights into pending questions. Cell. Reprogram. 2011, 13, 465. [Google Scholar] [CrossRef] [Green Version]
- Lv, F.; Lu, M.; Cheung, K.M.; Leung, V.Y.; Zhou, G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr. Stem Cell Res. Ther. 2012, 7, 389–399. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Cai, J.Q.; Lv, F.J.; Xie, H.L.; Yang, Z.M.; Huang, Y.C.; Deng, L. Species variation in the spontaneous calcification of bone marrow-derived mesenchymal stem cells. Cytotherapy 2013, 15, 323–329. [Google Scholar] [CrossRef]
- Lyu, F.J. Impact of Microenvironmental Changes during Degeneration on Intervertebral Disc Progenitor Cells: A Comparison with Mesenchymal Stem Cells. Bioengineering 2022, 9, 148. [Google Scholar] [CrossRef]
- Lyu, F.J.; Cheung, K.M.; Zheng, Z.; Wang, H.; Sakai, D.; Leung, V.Y. IVD progenitor cells: A new horizon for understanding disc homeostasis and repair. Nat. Rev. Rheumatol. 2019, 15, 102–112. [Google Scholar] [CrossRef]
- Martin-Rendon, E.; Sweeney, D.; Lu, F.; Girdlestone, J.; Navarrete, C.; Watt, S.M. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang 2008, 95, 137–148. [Google Scholar] [CrossRef]
- Öner, Ç.; Irmak, F.; Eken, G.; Öner, B.B.; Karsıdağ, S.H. The effect of stromal vascular fraction in an experimental frostbite injury model. Burns, 2022; in press. [Google Scholar] [CrossRef]
- van Dongen, J.A.; van Boxtel, J.; Uguten, M.; Brouwer, L.A.; Vermeulen, K.M.; Melenhorst, W.B.; Niessen, F.B.; Harmsen, M.C.; Stevens, H.P.; van der Lei, B. Tissue Stromal Vascular Fraction Improves Early Scar Healing: A Prospective Randomized Multicenter Clinical Trial. Aesthet. Surg. J. 2022, 42, P477–P488. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Gostelie, O.; Vonk, L.A.; De Bruijn, J.J.; Van Der Lei, B.; Harmsen, M.C.; Stevens, H.P. Fractionation of Adipose Tissue Procedure With a Disposable One-Hole Fractionator. Aesthet. Surg. J. 2020, 40, P194–P201. [Google Scholar] [CrossRef]
- Vriend, L.; van Dongen, J.A.; Pijpe, A.; Nieuwenhuis, M.K.; Jongen, S.; Harmsen, M.C.; van Zuijlen, P.; van der Lei, B. Stromal vascular fraction-enriched fat grafting as treatment of adherent scars: Study design of a non-randomized early phase trial. Trials 2022, 23, 575. [Google Scholar] [CrossRef]
- Park, S.S.; Park, M.; Lee, B.T. Autologous stromal vascular fraction-loaded hyaluronic acid/gelatin-biphasic calcium phosphate scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 132, 112533. [Google Scholar] [CrossRef] [PubMed]
- Sinno, S.; Wilson, S.; Brownstone, N.; Levine, S.M. Current Thoughts on Fat Grafting: Using the Evidence to Determine Fact or Fiction. Plast. Reconstr. Surg. 2016, 137, 818–824. [Google Scholar] [CrossRef]
- Tsekouras, A.; Mantas, D.; Tsilimigras, D.I.; Moris, D.; Kontos, M.; Zografos, G.C. Comparison of the Viability and Yield of Adipose-Derived Stem Cells (ASCs) from Different Donor Areas. Vivo 2017, 31, 1229–1234. [Google Scholar]
- Chaput, B.; Bertheuil, N.; Escubes, M.; Grolleau, J.L.; Garrido, I.; Laloze, J.; Espagnolle, N.; Casteilla, L.; Sensebe, L.; Varin, A. Mechanically Isolated Stromal Vascular Fraction Provides a Valid and Useful Collagenase-Free Alternative Technique: A Comparative Study. Plast. Reconstr. Surg. (1963) 2016, 138, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Banyard, D.A.; Salibian, A.A.; Widgerow, A.D.; Evans, G.R.D. Implications for human adipose-derived stem cells in plastic surgery. J. Cell. Mol. Med. 2015, 19, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Maeda Takiya, C.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Antiaging Treatment of the Facial Skin by Fat Graft and Adipose-Derived Stem Cells. Plast. Reconstr. Surg. (1963) 2015, 135, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Osinga, R.; Menzi, N.R.; Tchang, L.; Caviezel, D.; Kalbermatten, D.F.; Martin, I.; Schaefer, D.J.; Scherberich, A.; Largo, R.D. Effects of intersyringe processing on adipose tissue and its cellular components: Implications in autologous fat grafting. Plast. Reconstr. Surg. 2015, 135, 1618–1628. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- van Dijk, A.; Naaijkens, B.A.; Jurgens, W.J.F.M.; Nalliah, K.; Sairras, S.; van der Pijl, R.J.; Vo, K.; Vonk, A.B.A.; van Rossum, A.C.; Paulus, W.J.; et al. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011, 7, 219–229. [Google Scholar] [CrossRef]
- Semon, J.A.; Zhang, X.; Pandey, A.C.; Alandete, S.M.; Maness, C.; Zhang, S.; Scruggs, B.A.; Strong, A.L.; Sharkey, S.A.; Beuttler, M.M.; et al. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl. Med. 2013, 2, 789–796. [Google Scholar] [CrossRef]
- You, D.; Jang, M.J.; Kim, B.H.; Song, G.; Lee, C.; Suh, N.; Jeong, I.G.; Ahn, T.Y.; Kim, C.S. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl. Med. 2015, 4, 351–358. [Google Scholar] [CrossRef]
- Dai, L.G.; Huang, N.C.; Kang, L.Y.; Fu, K.Y.; Hsieh, P.S.; Dai, N.T. An In Vitro Study of the Effects of Mechanical and Enzymatic Isolation of Stromal Vascular Fraction on Wound Healing. Ann. Plast. Surg. 2022, 88 (Suppl. 1), S13–S21. [Google Scholar] [CrossRef]
- Dong, Z.; Peng, Z.; Chang, Q.; Lu, F. The survival condition and immunoregulatory function of adipose stromal vascular fraction (SVF) in the early stage of nonvascularized adipose transplantation. PLoS ONE 2013, 8, e80364. [Google Scholar] [CrossRef]
- Guerrero, J.; Dasen, B.; Frismantiene, A.; Pigeot, S.; Ismail, T.; Schaefer, D.J.; Philippova, M.; Resink, T.J.; Martin, I.; Scherberich, A. T-cadherin Expressing Cells in the Stromal Vascular Fraction of Human Adipose Tissue: Role in Osteogenesis and Angiogenesis. Stem Cells Transl. Med. 2022, 11, 213–229. [Google Scholar] [CrossRef]
- Dumont, N.A.; Rudnicki, M.A. Characterizing Satellite Cells and Myogenic Progenitors During Skeletal Muscle Regeneration; Springer: New York, NY, USA, 2017; pp. 179–188. [Google Scholar]
- Schultz, E.; Jaryszak, D.L.; Valliere, C.R. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 1985, 8, 217–222. [Google Scholar] [CrossRef]
- Banyard, D.A.; Sarantopoulos, C.N.; Borovikova, A.A.; Qiu, X.; Wirth, G.A.; Paydar, K.Z.; Haun, J.B.; Evans, G.; Widgerow, A.D. Phenotypic Analysis of Stromal Vascular Fraction after Mechanical Shear Reveals Stress-Induced Progenitor Populations. Plast. Reconstr. Surg. 2016, 138, 237e–247e. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.C.; Li, Z.; Li, J.; Lyu, F.J. Interaction between Stem Cells and the Microenvironment for Musculoskeletal Repair. Stem Cells Int. 2020, 2020, 7587428. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Boom in unproven cell therapies intensifies regulatory debate. Nature 2016, 537, 148. [Google Scholar] [CrossRef] [Green Version]
- Detiger, S.E.; Helder, M.N.; Smit, T.H.; Hoogendoorn, R.J. Adverse effects of stromal vascular fraction during regenerative treatment of the intervertebral disc: Observations in a goat model. Eur. Spine J. 2015, 24, 1992–2000. [Google Scholar] [CrossRef]









| cSVF | tSVF | |
|---|---|---|
| Composition | Cellular components (ADSC, pericytes, endothelial cells, etc.) | Cellular components (ADSC, pericytes, endothelial cells, etc.), ECM, cytokines |
| Digestive enzymes | Require | Not required |
| Time consumption | Long | Short |
| Security | Have side effects | Not found yet |
| Price | Expensive | Cheap |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; He, Z.; Li, S.; Wu, Z.; Tan, J.; Yang, W.; Li, G.; Pan, X.; Liu, Y.; Lyu, F.-J.; et al. The Effect of Tissue Stromal Vascular Fraction as Compared to Cellular Stromal Vascular Fraction to Treat Anal Sphincter Incontinence. Bioengineering 2023, 10, 32. https://doi.org/10.3390/bioengineering10010032
Chen W, He Z, Li S, Wu Z, Tan J, Yang W, Li G, Pan X, Liu Y, Lyu F-J, et al. The Effect of Tissue Stromal Vascular Fraction as Compared to Cellular Stromal Vascular Fraction to Treat Anal Sphincter Incontinence. Bioengineering. 2023; 10(1):32. https://doi.org/10.3390/bioengineering10010032
Chicago/Turabian StyleChen, Wenbin, Zijian He, Shuyu Li, Zixin Wu, Jin Tan, Weifeng Yang, Guanwei Li, Xiaoling Pan, Yuying Liu, Feng-Juan Lyu, and et al. 2023. "The Effect of Tissue Stromal Vascular Fraction as Compared to Cellular Stromal Vascular Fraction to Treat Anal Sphincter Incontinence" Bioengineering 10, no. 1: 32. https://doi.org/10.3390/bioengineering10010032
APA StyleChen, W., He, Z., Li, S., Wu, Z., Tan, J., Yang, W., Li, G., Pan, X., Liu, Y., Lyu, F. -J., & Li, W. (2023). The Effect of Tissue Stromal Vascular Fraction as Compared to Cellular Stromal Vascular Fraction to Treat Anal Sphincter Incontinence. Bioengineering, 10(1), 32. https://doi.org/10.3390/bioengineering10010032