A New Osteogenic Membrane to Enhance Bone Healing: At the Crossroads between the Periosteum, the Induced Membrane, and the Diamond Concept
Abstract
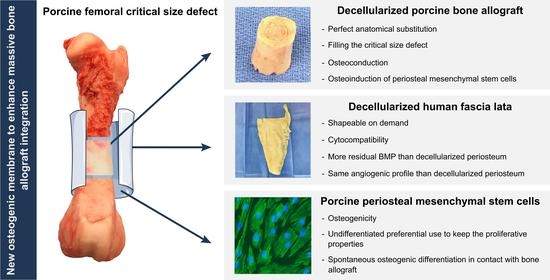
:1. Introduction
- Which best assembly of grafts should be used? Is there any combination required?
- How do tissue engineering processes influence the ECM outcomes from a growth factors (GFs) point of view? What happens to their angio- and/or osteoinductive potential?
- How the spontaneous differentiation of PMSCs towards the osteogenic lineage can be induced without any differentiation culture medium?
2. Materials and Methods
2.1. Tissue Harvesting and Processing
2.1.1. Human Fascia Lata (HFL) and Human Periosteum (HP)
2.1.2. Porcine Periosteal Mesenchymal Stem Cells (PMSCs) and Porcine Bone Allograft (PBA)
2.2. Histology
2.3. Immunohistochemistry (IHC)
2.4. Scanning Electron Microscopy (SEM)
2.5. Extracellular Matrix (ECM) and Cellular Components Quantification
2.6. Differentiation through Specific Differentiation Media
2.6.1. Chondrogenic Differentiation—Alcian Blue (AB) Staining
2.6.2. Adipogenic Differentiation—Oil Red O (ORO) Staining
2.6.3. Osteogenic Differentiation—Alizarin Red (AR) Staining
2.6.4. Cell Aggregate Creation
2.7. Recellularization
2.7.1. PMSC Seeding
2.7.2. Cell Growth—PrestoBlue
2.7.3. Cell Viability—Live/Dead
2.8. Differentiation through ECM
ECM Preparation
2.9. Growth Factors (GFs)—Immunoblot
2.9.1. Protein Extraction from ECM
2.9.2. Angiogenic GF Quantification
2.9.3. Bone Morphogenetic Protein (BMP) Quantification
2.10. Statistical Analyses
3. Results
3.1. Decellularization
3.2. PMSC Differentiation and Cell Aggregates
3.3. Recellularization and Cytocompatibility
3.4. Angio-Induction Potentials of ECMs
3.5. Osteoinduction Potentials of ECMs
3.5.1. BMP Content within the ECMs
3.5.2. Spontaneous In Vitro Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delloye, C.; Van Cauter, M.; Dufrane, D.; Francq, B.G.; Docquier, P.-L.; Cornu, O. Local Complications of Massive Bone Allografts: An Appraisal of Their Prevalence in 128 Patients. Acta Orthop. Belg. 2014, 80, 196–204. [Google Scholar] [PubMed]
- Moore, S.R.; Heu, C.; Yu, N.Y.; Whan, R.M.; Knothe, U.R.; Milz, S.; Knothe Tate, M.L. Translating Periosteum’s Regenerative Power: Insights From Quantitative Analysis of Tissue Genesis With a Periosteum Substitute Implant. Stem Cells Transl. Med. 2016, 5, 1739–1749. [Google Scholar] [CrossRef] [Green Version]
- Dalisson, B.; Charbonnier, B.; Aoude, A.; Gilardino, M.; Harvey, E.; Makhoul, N.; Barralet, J. Skeletal Regeneration for Segmental Bone Loss: Vascularised Grafts, Analogues and Surrogates. Acta Biomater. 2021, 136, 37–55. [Google Scholar] [CrossRef]
- Sen, M.K.; Miclau, T. Autologous Iliac Crest Bone Graft: Should It Still Be the Gold Standard for Treating Nonunions? Injury 2007, 38, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.F.; Chang, H.; Knothe Tate, M.L. Elucidating Multiscale Periosteal Mechanobiology: A Key to Unlocking the Smart Properties and Regenerative Capacity of the Periosteum? Tissue Eng. Part B Rev. 2013, 19, 147–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutelier, L.; Delloye, C. The Induction of Chondrogenesis after Fracture Repair. Int. Orthop. 1983, 7, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.C.; Bahney, C.S. Origin of Reparative Stem Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 490–503. [Google Scholar] [CrossRef]
- Perrin, S.; Colnot, C. Periosteal Skeletal Stem and Progenitor Cells in Bone Regeneration. Curr. Osteoporos. Rep. 2022, 20, 334–343. [Google Scholar] [CrossRef]
- Orwoll, E.S. Toward an Expanded Understanding of the Role of the Periosteum in Skeletal Health. J. Bone Miner. Res. 2003, 18, 949–954. [Google Scholar] [CrossRef]
- Dwek, J.R. The Periosteum: What Is It, Where Is It, and What Mimics It in Its Absence? Skelet. Radiol. 2010, 39, 319–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized Extracellular Matrix as an In Vitro Model to Study the Comprehensive Roles of the ECM in Stem Cell Differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masquelet, A.C. The Induced Membrane Technique. Orthop. Traumatol. Surg. Res. 2020, 106, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Alford, A.I.; Nicolaou, D.; Hake, M.; McBride-Gagyi, S. Masquelet’s Induced Membrane Technique: Review of Current Concepts and Future Directions. J. Orthop. Res. 2021, 39, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C.; Begue, T. The Concept of Induced Membrane for Reconstruction of Long Bone Defects. Orthop. Clin. N. Am. 2010, 41, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Andrzejowski, P.; Giannoudis, P.V. The “diamond Concept” for Long Bone Non-Union Management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Geng, J.; Gao, H.; Zhao, X.; Chen, J. Evaluations of Guided Bone Regeneration in Canine Radius Segmental Defects Using Autologous Periosteum Combined with Fascia Lata under Stable External Fixation. J. Orthop. Traumatol. 2015, 16, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Schubert, T.; Xhema, D.; Veriter, S.; Schubert, M.; Behets, C.; Delloye, C.; Gianello, P.; Dufrane, D. The Enhanced Performance of Bone Allografts Using Osteogenic-Differentiated Adipose-Derived Mesenchymal Stem Cells. Biomaterials 2011, 32, 8880–8891. [Google Scholar] [CrossRef]
- Chen, K.; Lin, X.; Zhang, Q.; Ni, J.; Li, J.; Xiao, J.; Wang, Y.; Ye, Y.; Chen, L.; Jin, K.; et al. Decellularized Periosteum as a Potential Biologic Scaffold for Bone Tissue Engineering. Acta Biomater. 2015, 19, 46–55. [Google Scholar] [CrossRef]
- van Steenberghe, M.; Schubert, T.; Guiot, Y.; Bouzin, C.; Bollen, X.; Gianello, P. Enhanced Vascular Biocompatibility of Decellularized Xeno-/Allogeneic Matrices in a Rodent Model. Cell Tissue Bank 2017, 18, 249–262. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Zhang, W.; Wang, H.; Li, J.; Pan, L.; Han, F.; Li, B. Biomimetic Periosteum-Bone Substitute Composed of Preosteoblast-Derived Matrix and Hydrogel for Large Segmental Bone Defect Repair. Acta Biomater. 2020, 113, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, B.; Wang, Z.; Li, Q.; Dai, C.; Wei, J. Progress of Periosteal Osteogenesis: The Prospect of In Vivo Bioreactor. Orthop. Surg. 2022, 14, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Song, J.; Zhu, G.; Li, X.; Liu, L.; Shi, X.; Wang, Y. Periosteum Tissue Engineering—A Review. Biomater. Sci. 2016, 4, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.E.; Nagle, R.; Soyster, M.; Song, L.; Tachibana, I.; Hathaway, J.K.; Powell, C.R. Robotic Sacral Colpopexy Using Autologous Fascia Lata Compared with Mesh. J. Endourol. 2021, 35, 801–807. [Google Scholar] [CrossRef]
- Song, Z.; Yang, D.; Yang, J.; Nie, X.; Wu, J.; Song, H.; Gu, Y. Abdominal Wall Reconstruction Following Resection of Large Abdominal Aggressive Neoplasms Using Tensor Fascia Lata Flap with or without Mesh Reinforcement. Hernia 2018, 22, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janik, S.; Hirtler, L.; Traxler, H.; Weninger, W.J.; Seemann, R.; Erovic, B.M. The Vascularized Fascia Lata Free Flap: An Anatomical Study and Clinical Considerations. Eur. Arch. Otorhinolaryngol. 2020, 277, 1733–1739. [Google Scholar] [CrossRef] [Green Version]
- Finn, M.A.; Faulkner, N.D.; Hetzel, S.J.; Anderson, P.A. Spinal Duraplasty Materials and Hydrostasis: A Biomechanical Study: Laboratory Investigation. SPI 2011, 15, 422–427. [Google Scholar] [CrossRef]
- Matthewson, G.; Coady, C.M.; Wong, I.H.-B. Rotator Cuff Reconstruction Using Fascia Lata Patch Autograft for the Nonrepairable Rotator Cuff Tear. Arthrosc. Tech. 2020, 9, e123–e130. [Google Scholar] [CrossRef] [Green Version]
- Sapino, G.; Zaugg, P.; Cherix, S.; Borens, O.; Lo, S.J.; Raffoul, W.; di Summa, P.G. ALT Flap with Vascularized Fascia Lata for One-Stage Functional Patellar Tendon Reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 467–476. [Google Scholar] [CrossRef]
- Ferretti, A.; Monaco, E.; Fabbri, M.; Mazza, D.; De Carli, A. The Fascia Lata Anterolateral Tenodesis Technique. Arthrosc. Tech. 2017, 6, e81–e86. [Google Scholar] [CrossRef]
- Fawzi-Grancher, S.; Goebbels, R.M.; Bigare, E.; Cornu, O.; Gianello, P.; Delloye, C.; Dufrane, D. Human Tissue Allograft Processing: Impact on in Vitro and in Vivo Biocompatibility. J. Mater. Sci. Mater. Med. 2009, 20, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Manon, J.; Evrard, R.; Maistriaux, L.; Fievé, L.; Heller, U.; Magnin, D.; Boisson, J.; Kadlub, N.; Schubert, T.; Lengelé, B.; et al. Periosteum and Fascia Lata: Are They so Different? Front. Bioeng. Biotechnol. 2022, 10, 944828. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a Periosteal Stem Cell Mediating Intramembranous Bone Formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Gangadaran, P.; Ranjan, R.; Jeyaraman, N.; Prajwal, G.S.; Mishra, P.C.; Rajendran, R.L.; Ahn, B.-C. Osteogenic and Chondrogenic Potential of Periosteum-Derived Mesenchymal Stromal Cells: Do They Hold the Key to the Future? Pharmaceuticals 2021, 14, 1133. [Google Scholar] [CrossRef] [PubMed]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum Contains Skeletal Stem Cells with High Bone Regenerative Potential Controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, A.; Perrin, S.; Martínez-Sarrà, E.; Kanagalingam, A.; Carvalho, C.; Luka, M.; Ménager, M.; Colnot, C. Skeletal Stem/Progenitor Cells in Periosteum and Skeletal Muscle Share a Common Molecular Response to Bone Injury. J. Bone Miner. Res. 2022, 37, 1545–1561. [Google Scholar] [CrossRef]
- Owston, H.E.; Ganguly, P.; Tronci, G.; Russell, S.J.; Giannoudis, P.V.; Jones, E.A. Colony Formation, Migratory, and Differentiation Characteristics of Multipotential Stromal Cells (MSCs) from “Clinically Accessible” Human Periosteum Compared to Donor-Matched Bone Marrow MSCs. Stem Cells Int. 2019, 2019, 6074245. [Google Scholar] [CrossRef] [Green Version]
- Marée, R.; Rollus, L.; Stévens, B.; Hoyoux, R.; Louppe, G.; Vandaele, R.; Begon, J.-M.; Kainz, P.; Geurts, P.; Wehenkel, L. Collaborative Analysis of Multi-Gigapixel Imaging Data Using Cytomine. Bioinformatics 2016, 32, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Nana, F.A.; Hoton, D.; Ambroise, J.; Lecocq, M.; Vanderputten, M.; Sibille, Y.; Vanaudenaerde, B.; Pilette, C.; Bouzin, C.; Ocak, S. Increased Expression and Activation of FAK in Small-Cell Lung Cancer Compared to Non-Small-Cell Lung Cancer. Cancers 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Duisit, J.; Amiel, H.; Wüthrich, T.; Taddeo, A.; Dedriche, A.; Destoop, V.; Pardoen, T.; Bouzin, C.; Joris, V.; Magee, D.; et al. Perfusion-Decellularization of Human Ear Grafts Enables ECM-Based Scaffolds for Auricular Vascularized Composite Tissue Engineering. Acta Biomater. 2018, 73, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Duisit, J.; Maistriaux, L.; Taddeo, A.; Orlando, G.; Joris, V.; Coche, E.; Behets, C.; Lerut, J.; Dessy, C.; Cossu, G.; et al. Bioengineering a Human Face Graft: The Matrix of Identity. Ann. Surg. 2017, 266, 754–764. [Google Scholar] [CrossRef]
- Rougier, G.; Maistriaux, L.; Fievé, L.; Xhema, D.; Evrard, R.; Manon, J.; Olszewski, R.; Szmytka, F.; Thurieau, N.; Boisson, J.; et al. Decellularized Vascularized Bone Grafts: A Preliminary in Vitro Porcine Model for Bioengineered Transplantable Bone Shafts. Front. Bioeng. Biotechnol. 2023, 10, 1003861. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Nauth, A.; Ristevski, B.; Li, R.; Schemitsch, E.H. Growth Factors and Bone Regeneration: How Much Bone Can We Expect? Injury 2011, 42, 574–579. [Google Scholar] [CrossRef]
- Ahlmann, E.; Patzakis, M.; Roidis, N.; Shepherd, L.; Holtom, P. Comparison of Anterior and Posterior Iliac Crest Bone Grafts in Terms of Harvest-Site Morbidity and Functional Outcomes. J. Bone Jt. Surg. Am. 2002, 84, 716–720. [Google Scholar] [CrossRef]
- Steffen, T.; Downer, P.; Steiner, B.; Hehli, M.; Aebi, M. Minimally Invasive Bone Harvesting Tools. Eur. Spine J. 2000, 9, S114–S118. [Google Scholar] [CrossRef] [Green Version]
- Phieffer, L.S.; Goulet, J.A. Delayed Unions of the Tibia. J. Bone Jt. Surg. Am. 2006, 88, 205–216. [Google Scholar] [CrossRef]
- Taylor, B.C.; French, B.G.; Fowler, T.T.; Russell, J.; Poka, A. Induced Membrane Technique for Reconstruction To Manage Bone Loss. J. Am. Acad. Orthop. Surg. 2012, 20, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, R.J.; Churchman, S.M.; Tan, H.B.; McGonagle, D.; Jones, E.; Giannoudis, P.V. Induced Periosteum a Complex Cellular Scaffold for the Treatment of Large Bone Defects. Bone 2013, 57, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of Stem Cell Viability in the Initial Healing Period in Rabbits with a Cranial Bone Defect According to the Type and Form of Scaffold. J. Periodontal Implant. Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T. The Effects of Processing Methods upon Mechanical and Biologic Properties of Porcine Dermal Extracellular Matrix Scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [Green Version]
- Uhl, F.E.; Zhang, F.; Pouliot, R.A.; Uriarte, J.J.; Enes, S.R.; Han, X.; Ouyang, Y.; Xia, K.; Westergren-Thorsson, G.; Malmström, A.; et al. Functional Role of Glycosaminoglycans in Decellularized Lung Extracellular Matrix. Acta Biomater. 2020, 102, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Salbach-Hirsch, J.; Rauner, M.; Hofbauer, C.; Hofbauer, L.C. New Insights into the Role of Glycosaminoglycans in the Endosteal Bone Microenvironment. Biol. Chem. 2021, 402, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Monier-Faugere, M.-C.; Langub, M.C.; Geng, Z.; Nakayama, T.; Pike, J.W.; Chernausek, S.D.; Rosen, C.J.; Donahue, L.-R.; Malluche, H.H.; et al. Targeted Overexpression of Insulin-Like Growth Factor I to Osteoblasts of Transgenic Mice: Increased Trabecular Bone Volume without Increased Osteoblast Proliferation. Endocrinology 2000, 141, 2674–2682. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and Death of Bone Cells: Basic Regulatory Mechanisms and Implications for the Pathogenesis and Treatment of Osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J. Fibroblast Growth Factor Signaling Controlling Osteoblast Differentiation. Gene 2003, 316, 23–32. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local Delivery of Insulin/IGF-1 for Bone Regeneration: Carriers, Strategies, and Effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef]
- Dicarlo, M.; Bianchi, N.; Ferretti, C.; Orciani, M.; Di Primio, R.; Mattioli-Belmonte, M. Evidence Supporting a Paracrine Effect of IGF-1/VEGF on Human Mesenchymal Stromal Cell Commitment. Cells Tissues Organs 2016, 201, 333–341. [Google Scholar] [CrossRef]
- Schwarz, S.; Gögele, C.; Ondruschka, B.; Hammer, N.; Kohl, B.; Schulze-Tanzil, G. Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction. IJMS 2019, 20, 1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauney, J.R.; Jaquiéry, C.; Volloch, V.; Heberer, M.; Martin, I.; Kaplan, D.L. In Vitro and in Vivo Evaluation of Differentially Demineralized Cancellous Bone Scaffolds Combined with Human Bone Marrow Stromal Cells for Tissue Engineering. Biomaterials 2005, 26, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical Applications of BMP-7/OP-1 in Fractures, Nonunions and Spinal Fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Even, J.; Eskander, M.; Kang, J. Bone Morphogenetic Protein in Spine Surgery: Current and Future Uses. J. Am. Acad. Orthop. Surg. 2012, 20, 547–552. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelin, E.; Jones, N.F.; Huang, J.I.; Brekke, J.H.; Lieberman, J.R. Healing of a Critical-Sized Defect in the Rat Femur with Use of a Vascularized Periosteal Flap, a Biodegradable Matrix, and Bone Morphogenetic Protein. J. Bone Jt. Surg. Am. 2005, 87, 1323–1331. [Google Scholar] [CrossRef]
- Ji, W.; Kerckhofs, G.; Geeroms, C.; Marechal, M.; Geris, L.; Luyten, F.P. Deciphering the Combined Effect of Bone Morphogenetic Protein 6 and Calcium Phosphate on Bone Formation Capacity of Periosteum Derived Cell-Based Tissue Engineering Constructs. Acta Biomater. 2018, 80, 97–107. [Google Scholar] [CrossRef]
- Badylak, S.; Freytes, D.; Gilbert, T. Extracellular Matrix as a Biological Scaffold Material: Structure and Function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Schubert, T.; Lafont, S.; Beaurin, G.; Grisay, G.; Behets, C.; Gianello, P.; Dufrane, D. Critical Size Bone Defect Reconstruction by an Autologous 3D Osteogenic-like Tissue Derived from Differentiated Adipose MSCs. Biomaterials 2013, 34, 4428–4438. [Google Scholar] [CrossRef]





| # | Antigen | Primary Antibody | Secondary Antibody | AF-Conjugated Tyramide | ||||
|---|---|---|---|---|---|---|---|---|
| Dilution | Company | Ref. | Polymer HRP | Company | Ref. | Fluorochrome | ||
| 1 | Col-1 | 1:500 | Abcam | ab34710 | Anti-rabbit | InvitroGen | B40955 | AF555 |
| 1 | RunX2 | 1:6000 | Cell signaling | 12556S | Anti-rabbit | InvitroGen | B40955 | AF555 |
| 2 | OC | 1:200 | Thermofisher | MA1-20786 | Anti-mouse | InvitroGen | B40958 | AF647 |
| Kruskal–Wallis | Mann–Whitney Post Hoc | |||||
|---|---|---|---|---|---|---|
| Dependent Variable | df | H | p-Value | Tissue 1 | Tissue 2 | p-Value |
| Angiogenin | 3 | 15.30 | 0.002 | D-PBA | D-HP | 0.012 a |
| D-HFL | 0.024 a | |||||
| EGF | 3 | 16.78 | 0.001 | D-PBA | D-HP | 0.012 a |
| D-HFL | D-HP | 0.012 a | ||||
| ENA-78 | 3 | 10.36 | 0.016 | |||
| β-FGF | 3 | 11.53 | 0.009 | D-PBA | D-HP | 0.024 a |
| GRO a/b/g | 3 | 13.85 | 0.003 | N-PBA | D-HP | 0.012 a |
| D-HFL | 0.012 a | |||||
| D-PBA | 0.012 a | |||||
| IFN-γ | 3 | 13.57 | 0.004 | N-PBA | D-HP | 0.012 a |
| D-HFL | 0.012 a | |||||
| D-PBA | 0.012 a | |||||
| IGF-1 | 3 | 16.29 | 0.001 | N-PBA | D-HFL | 0.012 a |
| D-PBA | 0.012 a | |||||
| IL-6 | 3 | 9.30 | 0.026 | |||
| IL-8 (CXCL8) | 3 | 13.10 | 0.004 | D-PBA | D-HP | 0.024 a |
| D-HFL | D-HP | 0.012 a | ||||
| Leptin | 3 | 12.19 | 0.007 | D-HFL | D-HP | 0.012 a |
| MCP-1 (CCL2) | 3 | 10.75 | 0.013 | |||
| PDGF-BB | 3 | 13.97 | 0.003 | N-PBA | D-HP | 0.012 a |
| D-HFL | 0.012 a | |||||
| D-PBA | 0.012 a | |||||
| PLGF | 3 | 9.16 | 0.027 | N-PBA | D-HFL | 0.012 a |
| RANTES (CCL5) | 3 | 11.82 | 0.008 | N-PBA | D-HFL | 0.012 a |
| D-PBA | 0.012 a | |||||
| TGF-β1 | 3 | 6.53 | 0.093 | |||
| TIMP-1 | 3 | 10.17 | 0.017 | |||
| TIMP-2 | 3 | 8.65 | 0.034 | N-PBA | D-HP | 0.012 a |
| D-HFL | 0.012 a | |||||
| TPO | 3 | 5.61 | 0.132 | |||
| VEGF-A | 3 | 4.13 | 0.248 | |||
| VEGF-D | 3 | 12.95 | 0.005 | N-PBA | D-HP | 0.012 a |
| D-HFL | 0.012 a | |||||
| Kruskal–Wallis | Mann–Whitney Post Hoc | |||||
|---|---|---|---|---|---|---|
| Dependent Variable | df | H | p-Value | Tissue 1 | Tissue 2 | p-Value |
| BMP-2 | 3 | 13.25 | 0.004 | D-PBA | D-HFL | 0.012 a |
| D-HP | 0.012 a | |||||
| BMP-4 | 3 | 3.07 | 0.381 | |||
| BMP-5 | 3 | 10.79 | 0.013 | D-PBA | D-HP | 0.012 a |
| BMP-6 | 3 | 3.33 | 0.343 | |||
| BMP-7 | 3 | 14.37 | 0.002 | D-PBA | D-HP | 0.012 a |
| D-HFL | D-HP | 0.012 a | ||||
| BMP-8 | 3 | 4.51 | 0.211 | |||
| BMP-9 | 3 | 5.78 | 0.123 | |||
| BMP-11 | 3 | 11.97 | 0.007 | D-PBA | D-HFL | 0.012 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manon, J.; Evrard, R.; Fievé, L.; Bouzin, C.; Magnin, D.; Xhema, D.; Darius, T.; Bonaccorsi-Riani, E.; Gianello, P.; Docquier, P.-L.; et al. A New Osteogenic Membrane to Enhance Bone Healing: At the Crossroads between the Periosteum, the Induced Membrane, and the Diamond Concept. Bioengineering 2023, 10, 143. https://doi.org/10.3390/bioengineering10020143
Manon J, Evrard R, Fievé L, Bouzin C, Magnin D, Xhema D, Darius T, Bonaccorsi-Riani E, Gianello P, Docquier P-L, et al. A New Osteogenic Membrane to Enhance Bone Healing: At the Crossroads between the Periosteum, the Induced Membrane, and the Diamond Concept. Bioengineering. 2023; 10(2):143. https://doi.org/10.3390/bioengineering10020143
Chicago/Turabian StyleManon, Julie, Robin Evrard, Lies Fievé, Caroline Bouzin, Delphine Magnin, Daela Xhema, Tom Darius, Eliano Bonaccorsi-Riani, Pierre Gianello, Pierre-Louis Docquier, and et al. 2023. "A New Osteogenic Membrane to Enhance Bone Healing: At the Crossroads between the Periosteum, the Induced Membrane, and the Diamond Concept" Bioengineering 10, no. 2: 143. https://doi.org/10.3390/bioengineering10020143
APA StyleManon, J., Evrard, R., Fievé, L., Bouzin, C., Magnin, D., Xhema, D., Darius, T., Bonaccorsi-Riani, E., Gianello, P., Docquier, P. -L., Schubert, T., Lengelé, B., Behets, C., & Cornu, O. (2023). A New Osteogenic Membrane to Enhance Bone Healing: At the Crossroads between the Periosteum, the Induced Membrane, and the Diamond Concept. Bioengineering, 10(2), 143. https://doi.org/10.3390/bioengineering10020143