Rapid Antigen Tests during the COVID-19 Era in Korea and Their Implementation as a Detection Tool for Other Infectious Diseases
Abstract
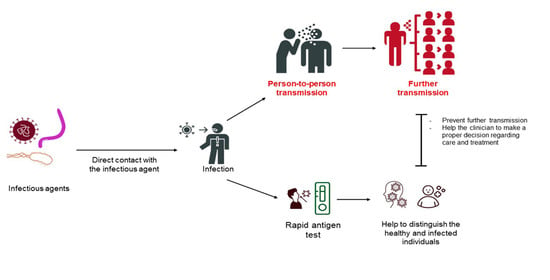
:1. Introduction
2. Database Search Strategy
3. Lateral Flow Immunoassay-Based Rapid Antigen Detection Test for COVID-19
4. Antigen Tests Amid the Battle against SARS-CoV-2 in Korea
4.1. Authorized Devices for SARS-CoV-2 Antigen Diagnostic Testing
4.1.1. STANDARDTM Q COVID-19 Ag Home Test
4.1.2. GenBody COVID-19 Ag
4.1.3. STANDARDTM F COVID-19 Ag FIA
4.1.4. BIOCREDIT COVID-19 Ag
4.1.5. Humasis COVID-19 Ag Test
4.1.6. CareStartTM COVID-19 Antigen Home Test
4.1.7. PanbioTM COVID-19 Ag Self-Test
4.2. RATs Are Complements of PCR for the Detection of SARS-CoV-2
5. RATs as a Detection Tool to Aid the Clinical Diagnosis of Other Infectious Diseases
5.1. Clinical Application of RATs for Respiratory Diseases Other than COVID-19
5.2. Clinical Application of RATs for Dengue Fever
5.3. Clinical Application of RATs for Malaria
5.4. Clinical Application of RATs for Sexually Transmitted Infection
6. Obstacles to the Application of RATs as a Detection Tool
7. Limitation of RATs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banoo, S.; Bell, D.; Bossuyt, P.; Herring, A.; Mabey, D.; Poole, F.; Smith, P.G.; Sriram, N.; Wongsrichanalai, C.; Linke, R. Evaluation of diagnostic tests for infectious diseases: General principles. Nat. Rev. Microbiol. 2007, 5, S21–S31. [Google Scholar] [CrossRef]
- Walker, D. Principles of diagnosis of infectious diseases. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Elsevier: Amsterdam, The Netherland, 2014; pp. 222–225. [Google Scholar] [CrossRef]
- John Hopkins Center for Health Security. Antigen Tests. Available online: https://www.centerforhealthsecurity.org/covid-19TestingToolkit/testing-basics/types-of-COVID-19-tests/diagnostic-tests/antigen-tests.html (accessed on 15 January 2023).
- Guglielmi, G. Rapid coronavirus tests: A guide for the perplexed. Nature 2021, 590, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Widyasari, K.; Yang, H.-R.; Jang, J.; Kang, T.; Kim, S. Evaluation of the Diagnostic Accuracy of Nasal Cavity and Nasopharyngeal Swab Specimens for SARS-CoV-2 Detection via Rapid Antigen Test According to Specimen Collection Timing and Viral Load. Diagnostics 2022, 12, 710. [Google Scholar] [CrossRef] [PubMed]
- CDC. Guidance for Antigen Testing for SARS-CoV-2 for Healthcare Providers Testing Individuals in the Community. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html (accessed on 14 January 2023).
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar]
- de Lourdes Moreno, M.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Carrio, A.; Sampedro, C.; Sanchez-Lopez, J.L.; Pimienta, M.; Campoy, P. Automated low-cost smartphone-based lateral flow saliva test reader for drugs-of-abuse detection. Sensors 2015, 15, 29569–29593. [Google Scholar] [CrossRef] [Green Version]
- Magambo, K.A.; Kalluvya, S.E.; Kapoor, S.W.; Seni, J.; Chofle, A.A.; Fitzgerald, D.W.; Downs, J.A. Utility of urine and serum lateral flow assays to determine the prevalence and predictors of cryptococcal antigenemia in HIV-positive outpatients beginning antiretroviral therapy in Mwanza, Tanzania. J. Int. AIDS Soc. 2014, 17, 19040. [Google Scholar] [CrossRef]
- Schramm, E.C.; Staten, N.R.; Zhang, Z.; Bruce, S.S.; Kellner, C.; Atkinson, J.P.; Kyttaris, V.C.; Tsokos, G.C.; Petri, M.; Connolly, E.S. A quantitative lateral flow assay to detect complement activation in blood. Anal. Biochem. 2015, 477, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S. How Do Rapid Antigen Tests Work? Available online: https://www.clinicallab.com/trends/covid-19-testing/how-do-rapid-antigen-tests-work-26279 (accessed on 4 January 2023).
- Pavia, C.S.; Plummer, M.M. The evolution of rapid antigen detection systems and their application for COVID-19 and other serious respiratory infectious diseases. J. Microbiol. Immunol. Infect. 2021, 54, 776–786. [Google Scholar] [CrossRef]
- Widyasari, K.; Kim, S.; Kim, S.; Lim, C.S. Performance Evaluation of STANDARD Q COVID/FLU Ag Combo for Detection of SARS-CoV-2 and Influenza A/B. Diagnostics 2023, 13, 32. [Google Scholar] [CrossRef]
- Frew, E.; Roberts, D.; Barry, S.; Holden, M.; Restell Mand, A.; Mitsock, E.; Tan, E.; Yu, W.; Skog, J. A SARS-CoV-2 antigen rapid diagnostic test for resource-limited settings. Sci. Rep. 2021, 11, 23009. [Google Scholar] [CrossRef] [PubMed]
- JOYSBIO. Coronavirus Antigen Rapid Test Kit. Available online: https://en.joysbio.com/covid-19-antigen-rapid-test-kit/ (accessed on 4 January 2023).
- Parvu, V.; Gary, D.S.; Mann, J.; Lin, Y.-C.; Mills, D.; Cooper, L.; Andrews, J.C.; Manabe, Y.C.; Pekosz, A.; Cooper, C.K. Factors that influence the reported sensitivity of rapid antigen testing for SARS-CoV-2. Front. Microbiol. 2021, 12, 714242. [Google Scholar] [CrossRef] [PubMed]
- FDA. In Vitro Diagnostics EUAs-Antigen Diagnostic Tests for SARS-CoV-2. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2#IndividualEUAs (accessed on 16 January 2023).
- Korean Ministry of Food and Drug Safety. Status of Domestic Official Approval of COVID-19 Diagnostic Reagent (as of 18 March 2021). Available online: https://mfds.go.kr/brd/m_74/view.do?seq=44004 (accessed on 16 January 2023).
- SD Biosensor. COVID-19 Ag Home Test. Available online: https://www.sdbiosensor.com/product/product_view?product_no=295 (accessed on 17 January 2023).
- Meridinan Bioscience. GenBody COVID-19 Ag. Available online: https://www.meridianbioscience.com/diagnostics/disease-areas/respiratory/coronavirus/genbody-covid-19-ag/ (accessed on 17 January 2023).
- SD Biosensor. STANDARD F COVID-19 Ag FIA. Available online: https://www.sdbiosensor.com/product/product_view?product_no=177 (accessed on 17 January 2023).
- Rapigen. Biocredit COVID-19 Ag Home Test Nasal. Available online: https://www.rapigen-inc.com/modules/catalogue_english/cg_view.html?cc=10&no=3 (accessed on 17 January 2023).
- Ture Healthcare. Humanis COVID-19 Home Test. Available online: https://truehealthcarethailand.com/en/product/humasis-atk-home-en (accessed on 17 January 2023).
- Korean Ministry of Food and Drug Safety. Status of Domestic Official Approval of COVID-19 Self-Test Kit (‘23.1.18.). Available online: https://mfds.go.kr/brd/m_74/view.do?seq=44513&srchFr=&srchTo=&srchWord=%EC%BD%94%EB%A1%9C%EB%82%9819+%EC%A7%84%EB%8B%A8%EC%8B%9C%EC%95%BD+%EA%B5%AD%EB%82%B4+%EC%A0%95%EC%8B%9D%ED%97%88%EA%B0%80+%ED%98%84%ED%99%A9&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1 (accessed on 17 January 2023).
- Rapid Test and Trace. Standard I-q COVID-19 AG Home Test. Available online: https://rapidtestandtrace.ca/standard-i-q-covid-19-ag-home-test/ (accessed on 1 February 2023).
- Global Health. MDx COVID-19 Ag Rapid Test. Available online: https://viaglobalhealth.com/product/mdx-covid-19-ag-rapid-test/ (accessed on 26 January 2023).
- Rapid Test and Trace. PCL COVID-19 Antigen Quick Test. Available online: https://rapidtestandtrace.ca/pcl-self-test-covid-19-ag/ (accessed on 26 January 2023).
- Access Bio. Access Bio’s CareStart™ COVID-19 Antigen. Available online: https://accessbio.net/products/covid-19-detection-kits/carestart-covid-19-antigen (accessed on 27 January 2023).
- Abbot. Panbio™ COVID-19 Antigen Self Test. Available online: https://www.globalpointofcare.abbott/en/product-details/panbio-covid-19-antigen-self-test.html (accessed on 28 January 2023).
- Boditech. Boditech Quick™ COVID-19 Ag Saliva. Available online: https://www.boditech.co.kr/en/product/reagents/id/932 (accessed on 29 January 2023).
- SG Medical. InstaView COVID-19 Antigen. Available online: https://www.sgmedical.kr/en/product/diagnostic-agent/rapid-test/instaview-covid-19-antigen/ (accessed on 30 January 2023).
- Oh, S.-M.; Jeong, H.; Chang, E.; Choe, P.G.; Kang, C.K.; Park, W.B.; Kim, T.S.; Kwon, W.Y.; Oh, M.-D.; Kim, N.J. Clinical application of the standard Q COVID-19 Ag Test for the detection of SARS-CoV-2 infection. J. Korean Med. Sci. 2021, 36, e101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.Y.; Huh, H.J.; Kim, N.; Sung, H.; Lee, H.; Roh, K.H.; Kim, T.S.; Hong, K.H. Clinical performance of the standard Q COVID-19 rapid antigen test and simulation of its real-world application in Korea. Ann. Lab. Med. 2021, 41, 588–592. [Google Scholar] [CrossRef]
- Kweon, O.J.; Lim, Y.K.; Kim, H.R.; Choi, Y.; Kim, M.-C.; Choi, S.-H.; Chung, J.-W.; Lee, M.-K. Evaluation of rapid SARS-CoV-2 antigen tests, AFIAS COVID-19 Ag, and ichroma COVID-19 Ag, with serial nasopharyngeal specimens from COVID-19 patients. PLoS ONE 2021, 16, e0249972. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Lee, S.; Widyasari, K.; Yi, J.; Bae, E.; Kim, S. Performance evaluation of STANDARD Q COVID-19 Ag home test for the diagnosis of COVID-19 during early symptom onset. J. Clin. Lab. Anal. 2022, 36, e24410. [Google Scholar] [CrossRef]
- Ristić, M.; Nikolić, N.; Čabarkapa, V.; Turkulov, V.; Petrović, V. Validation of the STANDARD Q COVID-19 antigen test in Vojvodina, Serbia. PLoS ONE 2021, 16, e0247606. [Google Scholar] [CrossRef]
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177. [Google Scholar] [CrossRef]
- Lopera, T.J.; Alzate-Ángel, J.C.; Díaz, F.J.; Rugeles, M.T.; Aguilar-Jiménez, W. The usefulness of antigen testing in predicting contagiousness in COVID-19. Microbiol. Spectr. 2022, 10, e0196221. [Google Scholar] [CrossRef]
- Lindner, A.K.; Nikolai, O.; Kausch, F.; Wintel, M.; Hommes, F.; Gertler, M.; Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2021, 57, 2003961. [Google Scholar] [CrossRef]
- Cerutti, F.; Burdino, E.; Milia, M.G.; Allice, T.; Gregori, G.; Bruzzone, B.; Ghisetti, V. Urgent need of rapid tests for SARS CoV-2 antigen detection: Evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J. Clin. Virol. 2020, 132, 104654. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynska, K.; Walory, J.; Charkiewicz, R.; Lewandowska, M.A.; Wasko, I.; Kozinska, A.; Majewski, P.; Baraniak, A. Clinical Validation of GenBody COVID-19 Ag, Nasal and Nasopharyngeal Rapid Antigen Tests for Detection of SARS-CoV-2 in European Adult Population. Biomedicines 2023, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Bal, J.; Seo, S.K.; Chong, C.-K.; Lee, J.H.; Park, H. Development and clinical evaluation of an immunochromatography-based rapid antigen test (GenBody™ COVAG025) for COVID-19 diagnosis. Viruses 2021, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, S.; Pablo-Marcos, D.; de la Fuente, S.V.; Rodríguez, M.J.R.; Gozalo, M.; Rodríguez-Lozano, J.; Méndez-Legaza, J.M.; Calvo, J. Evaluation of the rapid antigen detection test STANDARD F COVID-19 Ag FIA for diagnosing SARS-CoV-2: Experience from an Emergency Department. Diagn. Microbiol. Infect. Dis. 2022, 103, 115683. [Google Scholar] [CrossRef] [PubMed]
- Kiro, V.V.; Gupta, A.; Singh, P.; Sharad, N.; Khurana, S.; Prakash, S.; Dar, L.; Malhotra, R.; Wig, N.; Kumar, A. Evaluation of COVID-19 antigen fluorescence immunoassay test for rapid detection of SARS-CoV-2. J. Glob. Infect. Dis. 2021, 13, 91. [Google Scholar]
- Mak, G.C.; Cheng, P.K.; Lau, S.S.; Wong, K.K.; Lau, C.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020, 129, 104500. [Google Scholar] [CrossRef]
- Abdelrazik, A.M.; Elshafie, S.M.; Abdelaziz, H.M. Potential use of antigen-based rapid test for SARS-CoV-2 in respiratory specimens in low-resource settings in Egypt for symptomatic patients and high-risk contacts. Lab. Med. 2021, 52, e46–e49. [Google Scholar] [CrossRef]
- Bwogi, J.; Lutalo, T.; Tushabe, P.; Bukenya, H.; Eliku, J.P.; Ssewanyana, I.; Nabadda, S.; Nsereko, C.; Cotten, M.; Downing, R. Field evaluation of the performance of seven Antigen Rapid diagnostic tests for the diagnosis of SARS-CoV-2 virus infection in Uganda. PLoS ONE 2022, 17, e0265334. [Google Scholar] [CrossRef] [PubMed]
- Tabain, I.; Cucevic, D.; Skreb, N.; Mrzljak, A.; Ferencak, I.; Hruskar, Z.; Misic, A.; Kuzle, J.; Skoda, A.M.; Jankovic, H. Field evaluation of COVID-19 rapid antigen test: Are rapid antigen tests less reliable among the elderly? World J. Clin. Cases. 2022, 10, 6456. [Google Scholar] [CrossRef] [PubMed]
- Nóra, M.; Déri, D.; Veres, D.S.; Kis, Z.; Barcsay, E.; Pályi, B. Evaluating the field performance of multiple SARS-CoV-2 antigen rapid tests using nasopharyngeal swab samples. PLoS ONE 2022, 17, e0262399. [Google Scholar] [CrossRef]
- Pollock, N.R.; Tran, K.; Jacobs, J.R.; Cranston, A.E.; Smith, S.; O’Kane, C.Y.; Roady, T.J.; Moran, A.; Scarry, A.; Carroll, M. Performance and operational evaluation of the Access Bio CareStart Rapid Antigen Test in a high-throughput drive-through community testing site in Massachusetts. Proc. Open Forum Infect. Dis. 2021, 8, ofab243. [Google Scholar] [CrossRef] [PubMed]
- Sazed, S.A.; Kibria, M.G.; Zamil, M.F.; Hossain, M.S.; Khan, J.Z.; Juthi, R.T.; Hossain, M.E.; Ahmed, D.; Noor, Z.; Haque, R. Direct Nasal Swab for Rapid Test and Saliva as an Alternative Biological Sample for RT-PCR in COVID-19 Diagnosis. Microbiol. Spectr. 2022, 10, e0199822. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Matias, W.R.; Fulcher, I.R.; Molano, F.J.; Collins, S.; Uceta, E.; Zhu, J.; Paxton, R.M.; Gonsalves, S.F.; Harden, M.V. Evaluation of the Access Bio CareStart rapid SARS-CoV-2 antigen test in asymptomatic individuals tested a community mass-testing program in Western Massachusetts. Sci. Rep. 2022, 12, 21338. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.; Deslandes, V.; Smith, J.; Desjardins, M. Evaluation of the Abbott PanbioTM COVID-19 Ag rapid antigen test for the detection of SARS-CoV-2 in asymptomatic Canadians. Diagn. Microbiol. Infect. Dis. 2021, 101, 115514. [Google Scholar] [CrossRef]
- Ashagre, W.; Atnafu, A.; Wassie, L.; Tschopp, R.; Fentahun, D.; Assefa, G.; Wegayehu, T.; Wondale, B.; Mulu, A.; Miheret, A. Evaluation of the diagnostic performance of PanbioTM Abbott SARS-CoV-2 rapid antigen test for the detection of COVID-19 from suspects attending ALERT center. PLoS ONE 2022, 17, e0277779. [Google Scholar] [CrossRef]
- Merino, P.; Guinea, J.; Muñoz-Gallego, I.; González-Donapetry, P.; Galán, J.C.; Antona, N.; Cilla, G.; Hernáez-Crespo, S.; Díaz-de Tuesta, J.L.; Gual-de Torrella, A. Multicenter evaluation of the Panbio™ COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 758–761. [Google Scholar] [CrossRef]
- Eikelenboom-Boskamp, A.; den Ouden, M.; de Groot, T.; Stobernack, T.; Wertheim, H.; Voss, A. Evaluation of the Abbott PanbioTM COVID-19 antigen detection rapid diagnostic test among healthcare workers in elderly care. medrxiv 2022. preprint. [Google Scholar] [CrossRef]
- Scohy, A.; Anantharajah, A.; Bodéus, M.; Kabamba-Mukadi, B.; Verroken, A.; Rodriguez-Villalobos, H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020, 129, 104455. [Google Scholar] [CrossRef]
- Wells, C.R.; Pandey, A.; Moghadas, S.M.; Singer, B.H.; Krieger, G.; Heron, R.J.; Turner, D.E.; Abshire, J.P.; Phillips, K.M.; Michael Donoghue, A. Comparative analyses of eighteen rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation. Commun. Med. 2022, 2, 84. [Google Scholar] [CrossRef]
- Siddiqui, Z.K.; Chaudhary, M.; Robinson, M.L.; McCall, A.B.; Peralta, R.; Esteve, R.; Callahan, C.W.; Manabe, Y.C.; Campbell, J.D.; Johnson, J.K. Implementation and accuracy of BinaxNOW rapid antigen COVID-19 test in asymptomatic and symptomatic populations in a high-volume self-referred testing site. Microbiol. Spectr. 2021, 9, e01008–e01021. [Google Scholar] [CrossRef]
- Kohmer, N.; Toptan, T.; Pallas, C.; Karaca, O.; Pfeiffer, A.; Westhaus, S.; Widera, M.; Berger, A.; Hoehl, S.; Kammel, M. The comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J. Clin. Med. 2021, 10, 328. [Google Scholar] [CrossRef]
- Marimón, J.M.; Navarro-Marí, J.M. Rapid diagnostic test for respiratory infections. Enferm. Infecc. Microbiol Clin. 2017, 35, 108–115. [Google Scholar] [CrossRef]
- Cohen, J.F.; Bertille, N.; Cohen, R.; Chalumeau, M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst. Rev. 2016, 7, CD010502. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Arif, H.; Walsh, T. Community-acquired pneumonia secondary to Legionella pneumophila and Streptococcus pneumoniae: A rare co-infection. Cureus 2019, 11, e4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.Y.W.; Johnsson, A.T.A.; Ininbergs, K.; Athlin, S.; Özenci, V. Comparison of four streptococcus pneumoniae urinary antigen tests using automated readers. Microorganisms 2021, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Congestrì, F.; Morotti, M.; Vicari, R.; Pedna, M.; Sparacino, M.; Torri, A.; Bertini, S.; Fantini, M.; Sambri, V. Comparative evaluation of the novel IMMUNOCATCHTM Streptococcus pneumoniae (EIKEN CHEMICAL CO., LTD) test with the Uni-GoldTM Streptococcus pneumoniae assay and the BinaxNOW® Streptococcus pneumoniae antigen card for the detection of pneumococcal capsular antigen in urine samples. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 749–751. [Google Scholar]
- El-Khatib, Z.; Richter, L.; Ressler, J.; Benka, B. Diagnostic Study to Assess the Performance of a New Urinary Legionella Antigen Test—A National Study in Three Referral University Hospitals in Austria during 2014–2017. Int. J. Environ. Res. Public Health 2022, 19, 16705. [Google Scholar] [CrossRef]
- Badoux, P.; Kracht-Kosten, L.; Herpers, B.; Euser, S. Method Comparison of the ImmuView L. pneumophila and L. longbeachae Urinary Antigen Test with the BinaxNOW Legionella Urinary Antigen Card for Detection of Legionella pneumophila Serogroup 1 Antigen in Urine. J. Clin. Microbiol. 2020, 58, e01429. [Google Scholar] [CrossRef]
- Singh, L.; Grover, N. Detection of TB antigen by rapid test kit. Med. J. Armed Forces India 2011, 67, 196. [Google Scholar] [CrossRef] [Green Version]
- Van Pinxteren, L.A.; Ravn, P.; Agger, E.M.; Pollock, J.; Andersen, P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 2000, 7, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Aliannejad, R.; Bahrmand, A.; Abtahi, H.; Seifi, M.; Safavi, E.; Abdolrahimi, F.; Shahriaran, S. Accuracy of a new rapid antigen detection test for pulmonary tuberculosis. Iran. J. Microbiol. 2016, 8, 238. [Google Scholar] [PubMed]
- Gautam, H.; Singla, M.; Jain, R.; Lodha, R.; Kabra, S.; Singh, U. Point-of-care urine lipoarabinomannan antigen detection for diagnosis of tuberculosis in children. Int. J. Tuberc. Lung Dis. 2019, 23, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Akashi, Y.; Kiyasu, Y.; Terada, N.; Kurihara, Y.; Kato, D.; Miyazawa, T.; Muramatsu, S.; Shinohara, Y.; Ueda, A. A prospective evaluation of diagnostic performance of a combo rapid antigen test QuickNavi-Flu+ COVID19 Ag. J. Infect. Chemother. 2022, 28, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Kassim, F.M.; Izati, M.N.; TgRogayah, T.; Apandi, Y.M.; Saat, Z. Use of dengue NS1 antigen for early diagnosis of dengue virus infection. Southeast Asian J. Trop. Med. Public Health 2011, 42, 562–569. [Google Scholar]
- Paranavitane, S.A.; Gomes, L.; Kamaladasa, A.; Adikari, T.N.; Wickramasinghe, N.; Jeewandara, C.; Shyamali, N.L.A.; Ogg, G.S.; Malavige, G.N. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect. Dis. 2014, 14, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, I.; de Puig, H.; Hiley, M.; Carré-Camps, M.; Perdomo-Celis, F.; Narváez, C.F.; Salgado, D.M.; Senthoor, D.; O’Grady, M.; Phillips, E. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017, 9, eaan1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 2002, 15, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Beadle, C.; Long, G.W.; McElroy, P.; Hoffman, S.; Weiss, W.; Maret, S.; Oloo, A. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 1994, 343, 564–568. [Google Scholar] [CrossRef] [Green Version]
- Brenier-Pinchart, M.-P.; Pinel, C.; Croisonnier, A.; Brion, J.-P.; Faure, O.; Ponard, D.; Ambroise-Thomas, P. Diagnosis of malaria in non-endemic countries by the ParaSight-F test. Am. J. Trop. Med. Hyg. 2000, 63, 150–152. [Google Scholar] [CrossRef] [Green Version]
- Kodisinghe, H.; Perera, K.; Premawansa, S.; Naotunne, T.d.S.; Wickramasinghe, A.; Mendis, K. The ParaSight™-F dipstick test as a routine diagnostic tool for malaria in Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 398–402. [Google Scholar] [CrossRef]
- Humar, A.; Ohrt, C.; Harrington, M.A.; Pillai, D.; Kain, K.C. Para Sight® F Test Compared with the Polymerase Chain Reaction and Microscopy for the Diagnosis of Plasmodium falciparum Malaria in Travelers. Am. J. Trop. Med. Hyg. 1997, 56, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, P.; Mills, C.D.; Ohrt, C.; Harrington, M.A.; Kain, K.C. Comparison of the ParaSight™-F test and the ICT Malaria Pf™ test with the polymerase chain reaction for the diagnosis of Plasmodium falciparum malaria in travellers. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Fenton, K.; Lowndes, C. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex. Transm. Infect. 2004, 80, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Gewirtzman, A.; Bobrick, L.; Conner, K.; Tyring, S.K. Epidemiology of sexually transmitted infections. In Sexually Transmitted Infections and Sexually Transmitted Diseases; Gross, G., Tyring, S., Eds.; Springer: Berlin, Germany, 2011; pp. 13–34. [Google Scholar]
- WHO. Sexually Transmitted Infections (STIs). Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(STIs) (accessed on 20 February 2023).
- Greer, L.; Wendel, G.D., Jr. Rapid diagnostic methods in sexually transmitted infections. Infect. Dis. Clin. N. Am. 2008, 22, 601–617. [Google Scholar] [CrossRef]
- Adamson, P.C.; Loeffelholz, M.J.; Klausner, J.D. Point-of-care testing for sexually transmitted infections: A review of recent developments. Arch. Pathol. Lab. Med. 2020, 144, 1344–1351. [Google Scholar] [CrossRef]
- Savyon Diagnostics. QuickStripe™ Chlamydia Ag. Available online: https://www.savyondiagnostics.com/product/quickstripe-chlamydia-ag/ (accessed on 21 February 2023).
- Nilasari, H.; Ade Krisanti, R.I.; Rosana, Y.; Azizah, F. Diagnostic value of the QuickStripe™ chlamydia rapid test among high-risk women in Jakarta. Int. J. STD AIDS 2022, 33, 570–574. [Google Scholar] [CrossRef]
- Kelly, H.; Coltart, C.E.; Pai, N.P.; Klausner, J.D.; Unemo, M.; Toskin, I.; Peeling, R.W. Systematic reviews of point-of-care tests for the diagnosis of urogenital Chlamydia trachomatis infections. Sex. Transm. Infect. 2017, 93, S22–S30. [Google Scholar] [CrossRef] [Green Version]
- Ham, J.Y.; Jung, J.; Hwang, B.-G.; Kim, W.-J.; Kim, Y.-S.; Kim, E.-J.; Cho, M.-Y.; Hwang, M.-S.; Won, D.I.; Suh, J.S. Highly sensitive and novel point-of-care system, aQcare Chlamydia TRF kit for detecting Chlamydia trachomatis by using europium (Eu)(III) chelated nanoparticles. Ann. Lab. Med. 2015, 35, 50. [Google Scholar] [CrossRef] [Green Version]
- Samarawickrama, A.; Alexander, S.; Ison, C. A laboratory-based evaluation of the BioStar Optical ImmunoAssay point-of-care test for diagnosing Neisseria gonorrhoeae infection. J. Med. Microbiol. 2011, 60, 1779–1781. [Google Scholar] [CrossRef] [PubMed]
- Samarawickrama, A.; Cheserem, E.; Graver, M.; Wade, J.; Alexander, S.; Ison, C. Pilot study of use of the BioStar Optical ImmunoAssay GC point-of-care test for diagnosing gonorrhoea in men attending a genitourinary medicine clinic. J. Med. Microbiol. 2014, 63, 1111–1112. [Google Scholar] [CrossRef] [Green Version]
- Huppert, J.S.; Mortensen, J.E.; Reed, J.L.; Kahn, J.A.; Rich, K.D.; Miller, W.C.; Hobbs, M.M. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin. Infect. Dis. 2007, 45, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Klausner, J.; Coates, T.; Meza, R.; Gaydos, C.; Hardick, J.; Leon, S.; Caceres, C. Assessment of a rapid antigen detection system for Trichomonas vaginalis infection. Clin. Infect. Dis. 2003, 10, 1157–1158. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.B.; Kuldanek, K.; Muir, D.; Phekoo, K.; Black, A.; Sacks, R.; Smith, A.; Fidler, S. Clinical evaluation of the Determine HIV-1/2 Ag/Ab combo test. J. Infect. Dis. 2012, 206, 1947–1949. [Google Scholar] [CrossRef]
- Beelaert, G.; Fransen, K. Evaluation of a rapid and simple fourth-generation HIV screening assay for qualitative detection of HIV p24 antigen and/or antibodies to HIV-1 and HIV-2. J. Virol. Methods. 2010, 168, 218–222. [Google Scholar] [CrossRef]
- Mane, A.; Jain, S.; Jain, A.; Pereira, M.; Sirsat, A.; Pathak, G.; Bhoi, V.; Bhavsar, S.; Panda, S. Diagnostic performance of oral swab specimen for SARS-CoV-2 detection with rapid point-of-care lateral flow antigen test. Sci. Rep. 2022, 12, 7355. [Google Scholar] [CrossRef]
- Viloria Winnett, A.; Akana, R.; Shelby, N.; Davich, H.; Caldera, S.; Yamada, T.; Reyna, J.R.B.; Romano, A.E.; Carter, A.M.; Kim, M.K. Extreme differences in SARS-CoV-2 Omicron viral loads among specimen types drives poor performance of nasal rapid antigen tests for detecting presumably pre-infectious and infectious individuals, predicting improved performance of combination specimen antigen tests. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Kritikos, A.; Caruana, G.; Brouillet, R.; Miroz, J.-P.; Abed-Maillard, S.; Stieger, G.; Opota, O.; Croxatto, A.; Vollenweider, P.; Bart, P.-A. Sensitivity of rapid antigen testing and RT-PCR performed on nasopharyngeal swabs versus saliva samples in COVID-19 hospitalized patients: Results of a prospective comparative trial (RESTART). Microorganisms 2021, 9, 1910. [Google Scholar] [CrossRef]
- Robinson, J.L.; Lee, B.E.; Kothapalli, S.; Craig, W.R.; Fox, J.D. Use of throat swab or saliva specimens for detection of respiratory viruses in children. Clin. Infect. Dis. 2008, 46, e61–e64. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, J.M.d.O.; Roatt, B.M.; Vieira, P.M.d.A.; de Paiva, N.C.; Bernardes-Souza, B.; Lisboa, O.C.; Aguiar-Soares, R.D.d.O.; Reis, A.B.; Coura-Vital, W.; Carneiro, C.M. Performance of the Wondfo 2019-nCoV antigen test using self-collected nasal versus professional-collected nasopharyngeal swabs in symptomatic SARS-CoV-2 infection. Diagnosis 2022, 9, 398–402. [Google Scholar] [CrossRef]
- Van der Moeren, N.; Zwart, V.; Lodder, E.; Van den Bijllaardt, W.; Van Esch, H.; Stohr, J.; Pot, J.; Welschen, I.; Van Mechelen, P.; Pas, S. Evaluation of the test accuracy of a SARS-CoV-2 rapid antigen test in symptomatic community dwellung individuals in the Netherlands. PLoS ONE 2021, 16, e0250886. [Google Scholar] [CrossRef]
- Boum, Y.; Fai, K.N.; Nikolay, B.; Mboringong, A.B.; Bebell, L.M.; Ndifon, M.; Abbah, A.; Essaka, R.; Eteki, L.; Luquero, F. Performance and operational feasibility of antigen and antibody rapid diagnostic tests for COVID-19 in symptomatic and asymptomatic patients in Cameroon: A clinical, prospective, diagnostic accuracy study. Lancet Infect. Dis. 2021, 21, 1089–1096. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; Lippi, G. Association between viral load and positivization time of a SARS-CoV-2 rapid antigen test in routine nasopharyngeal specimens. J. Med. Biochem. 2022, 41, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.K.; Wong, K.K.; Mak, G.C.; Wong, A.H.; Ng, A.Y.; Chow, S.Y.; Lam, R.K.; Lau, C.; Ng, K.; Lim, W. Performance of laboratory diagnostics for the detection of influenza A (H1N1) v virus as correlated with the time after symptom onset and viral load. J. Clin. Virol. 2010, 47, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.E.; Riedel, S.; Dutta, S.; Arnaout, R.; Cheng, A.; Ditelberg, S.; Hamel, D.J.; Chang, C.A.; Kanki, P.J. SARS-CoV-2 antigen tests predict infectivity based on viral culture: Comparison of antigen, PCR viral load, and viral culture testing on a large sample cohort. Clin. Microbiol. Infect. 2023, 29, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Gillot, C.; Oliveira, M.; Cadrobbi, J.; Elsen, M.; Eucher, C.; Laffineur, K.; Rosseels, C.; Van Eeckhoudt, S.; Nicolas, J.-B. Head-to-head comparison of rapid and automated antigen detection tests for the diagnosis of SARS-CoV-2 infection. J. Clin. Med. 2021, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Pray, I.W.; Ford, L.; Cole, D.; Lee, C.; Bigouette, J.P.; Abedi, G.R.; Bushman, D.; Delahoy, M.J.; Currie, D.; Cherney, B. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September–October 2020. Morb. Mortal. Wkly. Rep. 2021, 69, 1642. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.L.; Mirza, A.; Gallagher, N.; Boudreau, A.; Garcia Jacinto, L.; Yu, T.; Norton, J.; Luo, C.H.; Conte, A.; Zhou, R. Limitations of molecular and antigen test performance for SARS-CoV-2 in symptomatic and asymptomatic COVID-19 contacts. J. Clin. Microbiol. 2022, 60, e0018722. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Zaboli, A.; Pfeifer, N.; Sibilio, S.; Tezza, G.; Bonora, A.; Ciccariello, L.; Ausserhofer, D. Rapid antigen test to identify COVID-19 infected patients with and without symptoms admitted to the Emergency Department. Am. J. Emerg. Med. 2022, 51, 92–97. [Google Scholar] [CrossRef]
- Maltha, J.; Gillet, P.; Jacobs, J. Malaria rapid diagnostic tests in endemic settings. Clin. Microbiol. Infect. 2013, 19, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Jian, M.-J.; Chung, H.-Y.; Chang, C.-K.; Lin, J.-C.; Yeh, K.-M.; Chen, C.-W.; Lin, D.-Y.; Chang, F.-Y.; Hung, K.-S.; Perng, C.-L. SARS-CoV-2 variants with T135I nucleocapsid mutations may affect antigen test performance. Int. J. Infect. Dis. 2022, 114, 112–114. [Google Scholar] [CrossRef]
- Bourassa, L.; Perchetti, G.A.; Phung, Q.; Lin, M.J.; Mills, M.G.; Roychoudhury, P.; Harmon, K.G.; Reed, J.C.; Greninger, A.L. A SARS-CoV-2 nucleocapsid variant that affects antigen test performance. J. Clin. Virol. 2021, 141, 104900. [Google Scholar] [CrossRef] [PubMed]
- Gyrolab SpinBlog, G. Solutions to Immunoassay Interference, Cross Reactivity and Other Challenges. Available online: https://www.gyrosproteintechnologies.com/spinblog/immunoassay-interference-crossreactivity-challenges (accessed on 10 January 2023).
- Australia National Health and Medical Research Council. Advice 26: Implementation of Rapid Antigen Testing in Various Settings. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.nhmrc.gov.au/sites/default/files/2023-02/NCHRAC-Advice-26-Implementation-of-RAT-in-various-settings-updated.pdf (accessed on 22 February 2023).
- CDC. Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 22 February 2023).
- Mouliou, D.S.; Gourgoulianis, K.I. False-positive and false-negative COVID-19 cases: Respiratory prevention and management strategies, vaccination, and further perspectives. Exp. Rev. Respir. Med. 2021, 15, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Feleke, S.M.; Reichert, E.N.; Mohammed, H.; Brhane, B.G.; Mekete, K.; Mamo, H.; Petros, B.; Solomon, H.; Abate, E.; Hennelly, C. Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat. Microbiol. 2021, 6, 1289–1299. [Google Scholar] [CrossRef]
- Osterman, A.; Badell, I.; Basara, E.; Stern, M.; Kriesel, F.; Eletreby, M.; Öztan, G.N.; Huber, M.; Autenrieth, H.; Knabe, R. Impaired detection of omicron by SARS-CoV-2 rapid antigen tests. Med. Microbiol. Iimmunol. 2022, 211, 105–117. [Google Scholar] [CrossRef]


| No. | RAT Kit Name | Sample Type | Ref. |
|---|---|---|---|
| 1 | STANDARDTM Q COVID-19 Ag Home Test | Nasal sample | [19,20] |
| 2 | GenBody COVID-19 Ag | Nasal sample | [19,21] |
| 3 | STANDARDTM F COVID-19 Ag FIA | Nasopharynx sample | [19,22] |
| 4 | BIOCREDIT COVID-19 Ag | Nasal sample | [19,23] |
| 5 | Humasis COVID-19 Ag Test | Nasopharyngeal or swab sample | [19,24] |
| 6 | STANDARDTM i-Q COVID-19 Ag Home Test | Nasal sample | [25,26] |
| 7 | MDx COVID-19 Ag Home Test | Nasopharyngeal sample | [25,27] |
| 8 | PCL SELF TEST-COVID-19 Ag | Saliva | [25,28] |
| 9 | CareStartTM COVID-19 Antigen Home Test | Nasal sample | [25,29] |
| 10 | PanbioTM COVID-19 Ag Self-Test | Nasal sample | [25,30] |
| 11 | Boditech QuickTM COVID-19 Ag Saliva | Saliva | [25,31] |
| 12 | InstaView COVID-19 Antigen Home Test | Nasopharyngeal sample | [25,32] |
| No. | Characteristics | RT-PCR | RAT |
|---|---|---|---|
| 1 | Type of analyte that is being assessed | Viral RNA | Viral protein (antigen) |
| 2 | Specimens | Nasopharyngeal swab, nasal swab, or saliva | Nasopharyngeal swab, nasal swab, or saliva |
| 3 | Sensitivity and specificity | ||
| Moderate | Low | |
| High | High | |
| Moderate | Moderate to low | |
| 4 | The complexity of the test | Requires complex procedures and specific instruments | Relatively easy to perform |
| 5 | Enables point-of-care testing | Some enable the point-of-care testing | Yes |
| 6 | Requires a well-trained person to conduct the test | Yes | No |
| 7 | Turnaround time | Varies, ranging from a few hours to 1 day | About 15–30 min |
| 8 | Suitable for screening | Varies; some may be suitable for screening purposes | Yes |
| 9 | Cost | Relatively high cost | Low cost |
| 10 | When should the test be used? |
|
|
| No. | Factors | Effect on the RAT | Ref. |
|---|---|---|---|
| 1 | Specimens |
| [98,99,100,101] |
| 2 | Sample condition | The RATs may perform poorly when being used to assess stored specimens rather than fresh specimens. | [5,46] |
| 3 | Sample collection | User-related errors that may occur during the RAT are related to the sample collection. An inaccurate sampling procedure due to a lack of experience may result in poor performance of the RAT. | [5,102] |
| 4 | Timing of test | RATs performed within the early days after symptom onset have high positive detection rates. | [5,103,104] |
| 5 | Viral load | A high viral load will yield a high detection rate; meanwhile, a lower viral load (insufficient for detection) will yield a low detection rate of the RAT. | [105,106,107] |
| 6 | Ct value | Higher Ct values tend to return poor detection rates of RATs. | [58,108] |
| 7 | Symptomatic/asymptomatic | RATs have a relatively poor performance when used for asymptomatic individuals. | [109,110,111] |
| 8 | RAT handling and storage | Locations with high humidity and temperature or windy conditions rapidly degrade the nitrocellulose capillary flow and thus may affect the performance of the RAT. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyasari, K.; Kim, S. Rapid Antigen Tests during the COVID-19 Era in Korea and Their Implementation as a Detection Tool for Other Infectious Diseases. Bioengineering 2023, 10, 322. https://doi.org/10.3390/bioengineering10030322
Widyasari K, Kim S. Rapid Antigen Tests during the COVID-19 Era in Korea and Their Implementation as a Detection Tool for Other Infectious Diseases. Bioengineering. 2023; 10(3):322. https://doi.org/10.3390/bioengineering10030322
Chicago/Turabian StyleWidyasari, Kristin, and Sunjoo Kim. 2023. "Rapid Antigen Tests during the COVID-19 Era in Korea and Their Implementation as a Detection Tool for Other Infectious Diseases" Bioengineering 10, no. 3: 322. https://doi.org/10.3390/bioengineering10030322
APA StyleWidyasari, K., & Kim, S. (2023). Rapid Antigen Tests during the COVID-19 Era in Korea and Their Implementation as a Detection Tool for Other Infectious Diseases. Bioengineering, 10(3), 322. https://doi.org/10.3390/bioengineering10030322