Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma
Abstract
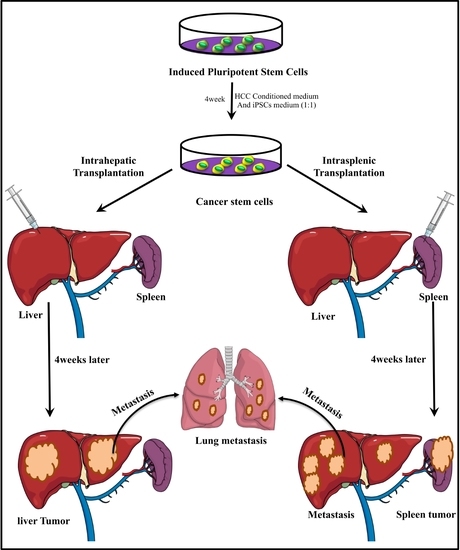
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal Expermints
2.3. RNA Extraction and RT-qPCR
2.4. Flow Cytometry
2.5. Histological Analysis
2.5.1. Hematoxylin and Eosin Staining
2.5.2. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Intrahepatic Transplantation
3.1.1. miPS-Huh7cm Cells Metastasized to the Lung
3.1.2. Characterization of miPS-Huh7cm Cells, Primary Tumor Derived Cells and Lung Metastatic Cells
3.2. IntraSplenic Transplantation
3.2.1. miPS-Huh7cm Cells Metastasized to Liver and Lung
3.2.2. Characterization of Primary Cells Derived from Spleen Tumor, Liver Metastatic Tumor and Lung Metastatic Nodules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSC | cancer stem cell |
| iPSCs | induced pluripotent stems |
| EMT | epithelial-to-mesenchyme transition |
| MET | mesenchyme-to-epithelial transition |
| LIF | leukemia inhibitory factor |
| miPS-Huh7cmPL cells | primary cultured cells isolated from liver tumor |
| LuMNL cells | lung metastatic nodule cells in case of intrahepatic transplantation |
| miPS-Huh7cmPS cells | primary culture derived from tumor in spleen |
| LiMN cells | cells derived from metastatic liver tumor |
| LuMNS cells | cells derived from metastatic lung nodules |
| GFP | green fluorescent protein |
| MEFs | mouse embryonic fibroblasts |
| MMP | matrix metalloproteinase |
References
- Sleeman, J.P. Metastasis: Understanding is the beginning of order in chaos. Semin. Cancer Biol. 2012, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Thompson, E.W.; Williams, E.D. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 2007, 185, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L.A.; Knuechel, R.; Kirchner, T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 2001, 98, 10356–10361. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Brennan, J.P.; Slavin, J.L.; Blick, T.; Thompson, E.W.; Williams, E.D. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: Role of fibroblast growth factor receptor-2. Cancer Res. 2006, 66, 11271–11278. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Ell, B.J.; Buffa, F.M.; Ibrahim, T.; Blanco, M.A.; Celià-Terrassa, T.; Mercatali, L.; Khan, Z.; Goodarzi, H.; Hua, Y.; et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 2011, 17, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Hugo, H.; Ackland, M.L.; Blick, T.; Lawrence, M.G.; Clements, J.A.; Williams, E.D.; Thompson, E.W. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J. Cell Physiol. 2007, 213, 374–383. [Google Scholar] [CrossRef]
- Robertson-Tessi, M.; Gillies, R.J.; Gatenby, R.A.; Anderson, A.R. Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res. 2015, 75, 1567–1579. [Google Scholar] [CrossRef]
- Ajani, J.A.; Song, S.; Hochster, H.S.; Steinberg, I.B. Cancer stem cells: The promise and the potential. Semin. Oncol. 2015, 42, S3–S17. [Google Scholar] [CrossRef] [PubMed]
- Dalerba, P.; Kalisky, T.; Sahoo, D.; Rajendran, P.S.; Rothenberg, M.E.; Leyrat, A.A.; Sim, S.; Okamoto, J.; Johnston, D.M.; Qian, D.; et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat. Biotechnol. 2011, 29, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Francia, G.; Cruz-Munoz, W.; Man, S.; Xu, P.; Kerbel, R.S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 2011, 11, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Kasai, T.; Li, Y.; Sugii, Y.; Jin, G.; Okada, M.; Vaidyanath, A.; Mizutani, A.; Satoh, A.; Kudoh, T.; et al. A model of cancer stem cells derived from mouse induced pluripotent stem cells. PLoS ONE 2012, 7, e33544. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Seno, M. Conversion of Stem Cells to Cancer Stem Cells: Undercurrent of Cancer Initiation. Cancers 2019, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Yan, T.; Calle, A.S.; Nair, N.; Hurley, L.; Kasai, T.; Kakuta, H.; Masuda, J.; Murakami, H.; Mizutani, A.; et al. iPSC-derived cancer stem cells provide a model of tumor vasculature. Am. J. Cancer Res. 2016, 6, 1906–1921. [Google Scholar] [PubMed]
- Nair, N.; Anna Sanchez Calle, A.S.; Zahra, M.H.; Prieto-Vila, M.; Oo, A.K.K.; Hurley, L.; Vaidyanath, A.; Seno, A.; Masuda, J.; Iwasaki, Y.; et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci. Rep. 2017, 7, 6838. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvasc. Res. 2007, 74, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, H.; Liyanarachchi, S.; Davuluri, R.V.; Auer, H.; Martin, E.W.; de la Chapelle, A.; Frankel, W.L. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene 2004, 23, 7366–7377. [Google Scholar] [CrossRef] [Green Version]
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007, 8, 444–448. [Google Scholar] [CrossRef]
- Campbell, L.L.; Polyak, K. Breast tumor heterogeneity: Cancer stem cells or clonal evolution? Cell Cycle 2007, 6, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Song, E.W. Research progress of solid tumor stem cells. J. SUN Yat-sen Univ. 2010, 31, 172–178. [Google Scholar]
- Stacker, S.A.; Baldwin, M.E.; Achen, M.G. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002, 16, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Deborah, R. Caswell and Charles Swanton. The role of tumor heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. 2017, 15, 133. [Google Scholar]
- Yan, T.; Mizutani, A.; Chen, L.; Takaki, M.; Hiramoto, Y.; Matsuda, S.; Shigehiro, T.; Kasai, T.; Kudoh, T.; Murakami, H.; et al. Characterization of Cancer Stem-Like Cells Derived from Mouse Induced Pluripotent Stem Cells Transformed by Tumor-Derived Extracellular Vesicles. J. Cancer 2014, 5, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Yan, T.; Mizutani, A.; Sota, T.; Hiramoto, Y.; Prieto-Vila, M.; Chen, L.; Satoh, A.; Kudoh, T.; Kasai, T.; et al. Cancer stem cells maintain a hierarchy of differentiation by creating their niche. Int. J. Cancer 2014, 135, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.S.; Nair, N.; Oo, A.K.; Prieto-Vila, M.; Koga, M.; Khayrani, A.C.; Hussein, M.; Hurley, L.; Vaidyanath, A.; Seno, A.; et al. A new PDAC mouse model originated from iPSCs-converted pancreatic stem cells (CSCcm). Am. J. Cancer Res. 2016, 6, 2799. [Google Scholar] [PubMed]
- Wu, W.; He, X.; Andayani, D.; Yang, L.; Ye, J.; Li, Y.; Chen, Y.; Li, L. Pattern of distant extrahepatic metastases in primary liver cancer: A SEER based study. J. Cancer 2017, 8, 2312–2318. [Google Scholar] [CrossRef]
- Castelli, G.; Pelosi, E.; Testa, U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers 2017, 9, 127. [Google Scholar] [CrossRef]
- Michelis, K.C.; Kitabayashi, A.N.; Lecce, L.; Franze´n, O.; Koplev, S.; Xu, Y.; Santini, M.P.; D’Escamard, V.; Lee, J.; Fuster, V.; et al. CD90 Identifies Adventitial Mesenchymal Progenitor Cells in Adult Human Medium- and Large-Sized Arteries Katherine, C. Stem Cell Rep. 2018, 11, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Muramaki, M.; Miyake, H.; Terakawa, T.; Kumano, M.; Sakai, I.; Fujisawa, M. Expression profile of E-cadherin and N-cadherin in non-muscle-invasive bladder cancer as a novel predictor of intravesical recurrence following transurethral resection. Urol. Oncol. 2012, 30, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, M.; Tsuji, K.; Yang, M.; Jiang, P.; Moossa, A.R.; Hoffman, R.M. In vivo color-codedimaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res. 2006, 66, 11293–11297. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.; Robidel, C.R.; Doghman-Bouguerra, M.; Atze, T.; Castellano, D.R.; Josselin, E.; Favier, J.; Lalli, E. Establishment of a mouse xenograft model of metastatic adrenocortical carcinoma. Oncotarget 2017, 8, 51050–51057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.Y.; Hong, H.K.; Ham, S.K.; Kim, C.I.; Cho, Y.B. Comparison of colorectal cancer in differentially established liver metastasis models. Anticancer Res. 2014, 34, 3321–3328. [Google Scholar] [PubMed]
- Rashidi, B.; Gamagami, R.; Sasson, A.; Sun, F.X.; Geller, J.; Moossa, A.R.; Hoffman, R.M. An orthotopic mouse model of remetastasis of human colon cancer liver metastasis. Clin. Cancer Res. 2000, 6, 2556–2561. [Google Scholar] [PubMed]
- Oliver, A.J.; Lau, P.K.H.; Unsworth, A.S.; Loi, S.; Darcy, P.K.; Kershaw, M.H.; Slaney, C.Y. Tissue-Dependent Tumor Microenvironments and Their Impact on Immunotherapy Responses. Front. Immunol. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wu, M.C.; Sham, J.S.; Zhang, W.; Wu, W.Q.; Guan, X.Y. Prognostic significance of c-myc and aib1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer 2002, 95, 2346–2352. [Google Scholar] [CrossRef] [PubMed]









| Gene | NM | Forward Primer | Reverse Primer |
|---|---|---|---|
| GAPDH | NM_008084 | AACGGCACAGTCAAGGCCGA | ACCCTTTTGGCTCCACCCTT |
| Nanog | NM_028016.3 | AGGGTCTGCTACTGAGATGCTCTG | CAACCACTGGTTTTTCTGCCACCG |
| Klf4 | NM_010637.3 | GGACTTACAAAATGCCAAGGGGTG | TCGCTTCCTCTTCCTCCGACACA |
| CD44 | NM_009851.2 | AGAAAAATGGCCGCTACAGTATC | TGCATGTTTCAAAACCCTTGC |
| CD90 | NM_009382.3 | TGCAGCTAGGGGAGTCCAGAAT | TCCAGGCGAAGGTTTTGGTT |
| ALDH1 | NM_001361503.1 | AACACAGGTTGGCAAGTTAATCA | TGCGACACAACATTGGCCTT |
| Slug | NM_011415.3 | CACACACACAGTTATTATTTCCCCA | ACTTACACGCCCCAAGGATG |
| Twist1 | NM_011658.2 | GCCGGAGACCTAGATGTCATTGT | TTAAAAGTGTGCCCCACGCC |
| E-cadherin | NM_009864.3 | AACCCAAGCACGTATCAGGG | GGGGTCTGTGACAACAACGA |
| N-cadherin | NM_007664.5 | CCTTGCTTCAGGCGTCTGTG | CTTGAAATCTGCTGGCTCGC |
| Vimentin | NM_011701.4 | GCCCTTAAAGGCACTAACGAG | ATTCACGAAGGTGACGAGCC |
| Fibronectin | NM_010233.2 | CCAACCTCTTGGTGCGCTA | AATCGAGACCTGTTTTCTGCCT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afify, S.M.; Hassan, G.; Osman, A.; Calle, A.S.; Nawara, H.M.; Zahra, M.H.; EL-Ghlban, S.; Mansour, H.; Alam, M.J.; Abu Quora, H.A.; et al. Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma. Bioengineering 2019, 6, 73. https://doi.org/10.3390/bioengineering6030073
Afify SM, Hassan G, Osman A, Calle AS, Nawara HM, Zahra MH, EL-Ghlban S, Mansour H, Alam MJ, Abu Quora HA, et al. Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma. Bioengineering. 2019; 6(3):73. https://doi.org/10.3390/bioengineering6030073
Chicago/Turabian StyleAfify, Said M., Ghmkin Hassan, Amira Osman, Anna Sanchez Calle, Hend M Nawara, Maram Hussein Zahra, Samah EL-Ghlban, Hager Mansour, Md Jahangir Alam, Hagar A Abu Quora, and et al. 2019. "Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma" Bioengineering 6, no. 3: 73. https://doi.org/10.3390/bioengineering6030073
APA StyleAfify, S. M., Hassan, G., Osman, A., Calle, A. S., Nawara, H. M., Zahra, M. H., EL-Ghlban, S., Mansour, H., Alam, M. J., Abu Quora, H. A., Du, J., Seno, A., Iwasaki, Y., & Seno, M. (2019). Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma. Bioengineering, 6(3), 73. https://doi.org/10.3390/bioengineering6030073