Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering
Abstract
:1. Oxidative Stress in Tissue Engineering: The Rationale for Antioxidant Integration
2. Natural Antioxidants: An Overview
3. Biomaterials: Overview of Natural Polymers
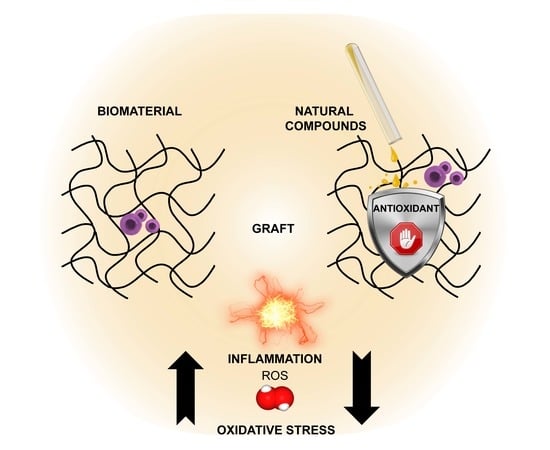
4. New Opportunities for Natural Antioxidant Combination with Tissue Engineering
4.1. Oxidative Stress Reduction Ex Vivo and In Vivo
4.2. Local Delivery
4.3. Biodegradability and Biomechanical Properties
4.4. Biocompatibility
4.5. Immunomodulation and Pathogen Defense
5. Towards Therapeutic Application: Antioxidant Biomaterials under Investigation
5.1. Wound Healing
5.2. Bone Tissue Engineering
5.3. Cardiovascular Tissue Engineering
5.4. Neural Tissue Engineering and Other Tissue Application
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3T3 | Swiss albino mouse fibroblast (cell line clone) |
| 3T6 | Swiss albino mouse fibroblasts (cell line clone) |
| α-SMA | alpha smooth muscle actin |
| ALG | alginate |
| AST | astaxanthin |
| AV | Aloe vera |
| ARE | antioxidant response element |
| bFGF | basic fibroblast growth factor |
| AD-MSC (s) | adipose (derived) mesenchymal/stromal stem cell(s) |
| BM-MSC (s) | bone marrow mesenchymal/stromal stem cell(s) |
| CAT | catalase |
| CH | chitosan |
| CHO | Chinese hamster ovary (cells) |
| CO | Calendula Officinalis |
| COX-2 | cyclooxygenase-2 |
| Cur | curcumin |
| Eahy926 | human umbilical vein endothelial cells (hybrid cell line) |
| EGCG | epigallocatechin gallate |
| E.O. | essential oil |
| ECM | extra cellular matrix |
| GF | growth factors |
| GMP | good manufacturing practices |
| GSH-Px | glutathione peroxidase |
| GVHD | graft versus host disease |
| HA | hyaluronic acid |
| Hap | hydroxyapatite |
| HFF1 | human foreskin fibroblast (cell line) |
| HUVEC | human umbilical vein endothelial cells |
| IL-1 | interleukin-1 |
| IL-1b | interleukin 1 beta |
| iPSC | induced pluripotent stem cells |
| Keap1 | Kelch-like ECH-associated protein 1 |
| L929 | mouse subcutaneous connective tissue fibroblast (cell line) |
| LPS | lipopolysaccharides |
| M1 | Th1-response macrophage phenotype (classically activated) |
| M2 | Th2-response macrophage phenotype (alternatively activated) |
| MC3T3-E1 | mouse preosteoblast (cell line) |
| MG63 | human osteosarcoma (cell line) |
| MICH | multiresponsive catechol-Fe3+ coordination hydrogel |
| MO | Moringa oleifera |
| MMP | matrix metalloproteinases |
| mRNA | messenger ribonucleic acid |
| MSC | mesenchymal stem cells |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-kB | nuclear factor kappa-B |
| NIH3T3 | mouse swiss NIH embryo |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NOS | nitric oxide synthases |
| NOX | NADPH oxidase |
| P(3HB-co-3HV) | poly(3-hydroxybutyrate-co-3- hydroxyvalerate) |
| PBAE | poly(β-amino ester) |
| PA | proanthocyanidins |
| PA-6 | polyammide 6 |
| PC12 | pheochromocytoma (cell line) |
| PCL | polycaprolactone |
| PDMS | polydimethylsiloxane |
| PDX | polydioxanone |
| PE | polyethylene |
| PEG | polyethylene glycol |
| PHBV | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | polylactic acid |
| PLGA | poly(lactic-co-glycolic acid) |
| PMAA | polymethacrylic acid |
| PS | polystyrene |
| PU | polyuretane |
| PVA | polyvinyl alcohol |
| PVP | polyvinylpyrrolidone |
| Qu | quercetin |
| RA | rosmarinic acid |
| RAW264.7 | Abelson murine leukemia virus transformed (cell line) |
| ROS | reactive oxygen species |
| SaOS2 | human osteosarcoma (cell line) |
| SOD | superoxide dismutase |
| SF | silk fibroin |
| TA | tannic acid |
| TGF-β | transforming growth factor beta |
| TNF-α | tumor necrosis factor alpha |
| SHSY-5Y | human neuroblastoma (cell line) |
| UMR106 | rat osteosarcoma (cell line) |
| VEGF | vascular endothelial growth factor |
References
- Almouemen, N.; Kelly, H.M.; O’Leary, C. Tissue Engineering: Understanding the Role of Biomaterials and Biophysical Forces on Cell Functionality Through Computational and Structural Biotechnology Analytical Methods. Comput. Struct. Biotechnol. J. 2019, 17, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Roche, C.D.; Brereton, R.J.L.; Ashton, A.W.; Jackson, C.; Gentile, C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur. J. CardioThoracic Surg. 2020, 58, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Fujita, B.; Zimmermann, W.-H. Myocardial Tissue Engineering for Regenerative Applications. Curr. Cardiol. Rep. 2017, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [Green Version]
- Perez-Basterrechea, M.; Esteban, M.M.; Vega, J.A.; Obaya, A.J. Tissue-engineering approaches in pancreatic islet transplantation. Biotechnol. Bioeng. 2018, 115, 3009–3029. [Google Scholar] [CrossRef]
- Salg, G.A.; Giese, N.A.; Schenk, M.; Hüttner, F.J.; Felix, K.; Probst, P.; Diener, M.K.; Hackert, T.; Kenngott, H.G. The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells. J. Tissue Eng. 2019, 10, 204173141988470. [Google Scholar] [CrossRef]
- Shakiba, N.; Zandstra, P.W. Engineering cell fitness: Lessons for regenerative medicine. Curr. Opin. Biotechnol. 2017, 47, 7–15. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Freschi, M.; Lorenzini, A.; Prata, C.; Maraldi, T.; Hrelia, S. Combination of epigallocatechin gallate and sulforaphane counteracts in vitro oxidative stress and delays stemness loss of amniotic fluid stem cells. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Lee, D.; Song, C.G.; Kang, P.M. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 2015, 10, 2709–2723. [Google Scholar] [CrossRef]
- Shi, S.; Xue, F. Current Antioxidant Treatments in Organ Transplantation. Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Shen, J. Hydrogen, a potential safeguard for graft-versus-host disease and graft ischemia-reperfusion injury? Clinics 2016, 71, 544–549. [Google Scholar] [CrossRef]
- Cannistrà, M.; Ruggiero, M.; Zullo, A.; Gallelli, G.; Serafini, S.; Maria, M.; Naso, A.; Grande, R.; Serra, R.; Nardo, B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 2016, 33, S57–S70. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battula, N.R.; Andreoni, K.A. Oxygenated Preservation Solutions for Organ Preservation. Transplantation 2019, 103, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; Grocott, M.P.W. Oxygen therapy and anaesthesia: Too much of a good thing? Anaesthesia 2015, 70, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Jin, L.; Ren, K.; Xu, Q.; Hong, T.; Wu, S.; Zhang, Y.; Wang, Z. Multifunctional carbon dots for live cell staining and tissue engineering applications. Polym. Compos. 2018, 39, 73–80. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008. [Google Scholar] [CrossRef]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in Exercise Performance and Prevention of Exercise-Induced Muscle Damage. Oxid. Med. Cell. Longev. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem. Rev. 2010, 9, 147–170. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Kim, I.-S.; More, S.V.; Kim, B.-W.; Choi, D.-K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.-H. Naturally Occurring NF-κB Inhibitors. Mini-Reviews Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef]
- Maraldi, T. Natural compounds as modulators of NADPH oxidases. Oxid. Med. Cell. Longev. 2013, 2013, 271602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Hrelia, S. Combined Treatment with Three Natural Antioxidants Enhances Neuroprotection in a SH-SY5Y 3D Culture Model. Antioxidants 2019, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Shafi, S.; Ansari, H.R.; Bahitham, W.; Aouabdi, S. The Impact of Natural Antioxidants on the Regenerative Potential of Vascular Cells. Front. Cardiovasc. Med. 2019, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Khanna, S.; Gordillo, G.; Bagchi, D.; Bagchi, M.; Roy, S. Oxygen, Oxidants, and Antioxidants in Wound Healing. Ann. N. Y. Acad. Sci. 2002, 957, 239–249. [Google Scholar] [CrossRef]
- Palacio, C.; Mooradian, A.D. Clinical trials and antioxidant outcomes. In Oxidative Stress and Antioxidant Protection; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 493–506. [Google Scholar]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative Stress in Cell Death and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [Green Version]
- Ohlow, M.J.; Sohre, S.; Granold, M.; Schreckenberger, M.; Moosmann, B. Why Have Clinical Trials of Antioxidants to Prevent Neurodegeneration Failed?—A Cellular Investigation of Novel Phenothiazine-Type Antioxidants Reveals Competing Objectives for Pharmaceutical Neuroprotection. Pharm. Res. 2017, 34, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative Stress in Alzheimer’s Disease: Why Did Antioxidant Therapy Fail? Oxid. Med. Cell. Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Kabirian, F.; Mozafari, M. Decellularized ECM-derived bioinks: Prospects for the future. Methods 2020, 171, 108–118. [Google Scholar] [CrossRef]
- Rieder, E.; Steinacher-Nigisch, A.; Weigel, G. Human immune-cell response towards diverse xenogeneic and allogeneic decellularized biomaterials. Int. J. Surg. 2016, 36, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Varma, R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019, 21, 4839–4867. [Google Scholar] [CrossRef]
- Phan, N.V.; Wright, T.; Rahman, M.M.; Xu, J.; Coburn, J.M. In Vitro Biocompatibility of Decellularized Cultured Plant Cell-Derived Matrices. ACS Biomater. Sci. Eng. 2020, 6, 822–832. [Google Scholar] [CrossRef]
- Lapomarda, A.; De Acutis, A.; Chiesa, I.; Fortunato, G.M.; Montemurro, F.; De Maria, C.; Mattioli Belmonte, M.; Gottardi, R.; Vozzi, G. Pectin-GPTMS-Based Biomaterial: Toward a Sustainable Bioprinting of 3D scaffolds for Tissue Engineering Application. Biomacromolecules 2020, 21, 319–327. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, N.; Park, S.H.; Ju, J.H. Induced Osteogenesis in Plants Decellularized Scaffolds. Sci. Rep. 2019, 9, 20194. [Google Scholar] [CrossRef] [PubMed]
- Cassimjee, H.; Kumar, P.; Choonara, Y.E.; Pillay, V. Proteosaccharide combinations for tissue engineering applications. Carbohydr. Polym. 2020, 235, 115932. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Quarta, A.; Marradi, M.; Ragusa, A. Recent Developments in the Reduction of Oxidative Stress through Antioxidant Polymeric Formulations. Pharmaceutics 2019, 11, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.; Carter, P.; Bhattarai, N. Aloe Vera for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Agnes Mary, S.; Giri Dev, V.R. Electrospun herbal nanofibrous wound dressings for skin tissue engineering. J. Text. Inst. 2015, 106, 886–895. [Google Scholar] [CrossRef]
- López, Z.; Femenia, A.; Núñez-Jinez, G.; Salazar Zúñiga, M.N.; Cano, M.E.; Espino, T.; Knauth, P. In Vitro Immunomodulatory Effect of Food Supplement from Aloe vera. Evid. Based Complement. Altern. Med. 2019. [Google Scholar] [CrossRef] [Green Version]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull. Natl. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef] [Green Version]
- Ji, A.; Zhang, S.; Bhagia, S.; Yoo, C.G.; Ragauskas, A.J. 3D printing of biomass-derived composites: Application and characterization approaches. RSC Adv. 2020, 10, 21698–21723. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020, 10, 175. [Google Scholar] [CrossRef] [Green Version]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Davoudi, Z.; Xing, X.; Yu, X.; Cheng, X.; Li, Z.; Deng, H.; Wang, Q. Advanced Silk Fibroin Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715. [Google Scholar] [CrossRef]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro- and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Zhao, L.; Mehwish, H.M.; Wu, Y.; Mahmood, S. Chitosan and its derivatives: Synthesis, biotechnological applications, and future challenges. Appl. Microbiol. Biotechnol. 2019, 103, 1557–1571. [Google Scholar] [CrossRef]
- Francesko, A.; Tzanov, T. Chitin, Chitosan and Derivatives for Wound Healing and Tissue Engineering. Adv. Biochem. Eng. Biotechnol. 2010, 125, 1–27. [Google Scholar]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Kim, C.-H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 475–485. [Google Scholar]
- Moran, H.B.T.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. Biores. Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [Green Version]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K.; Singh, S.M.; Arora, A.P.; Implant, J.K. Implant biomaterials: A comprehensive review. A Compr. Rev. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving Controlled Biomolecule—Biomaterial Conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Wang, Z.; Ding, J.; Wang, S.; Huang, B.; Ke, S.; Gao, C. Reactive oxygen species (ROS)-responsive biomaterials mediate tissue microenvironments and tissue regeneration. J. Mater. Chem. B 2019, 7, 5019–5037. [Google Scholar] [CrossRef] [PubMed]
- Lakes, A.L.; Jordan, C.T.; Gupta, P.; Puleo, D.A.; Hilt, J.Z.; Dziubla, T.D. Reducible disulfide poly(beta-amino ester) hydrogels for antioxidant delivery. Acta Biomater. 2018, 68, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Sthijns, M.M.J.P.E.; van Blitterswijk, C.A.; LaPointe, V.L.S. Redox regulation in regenerative medicine and tissue engineering: The paradox of oxygen. J. Tissue Eng. Regen. Med. 2018, 12, 2013–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levato, R.; Jungst, T.; Scheuring, R.G.; Blunk, T.; Groll, J.; Malda, J. From Shape to Function: The Next Step in Bioprinting. Adv. Mater. 2020, 32, 1906423. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F. Antibacterial activities and antioxidant capacity of Aloe vera. Org. Med. Chem. Lett. 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; da Ribeiro Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; García, A.J. Surface Modification of Biomaterials. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 651–660. [Google Scholar] [CrossRef]
- Gillispie, G.J.; Park, J.; Copus, J.S.; Asari, A.K.P.R.; Yoo, J.J.; Atala, A.; Lee, S.J. Three-Dimensional Tissue and Organ Printing in Regenerative Medicine. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 831–852. [Google Scholar] [CrossRef]
- Choi, G.; Cha, H.J. Recent advances in the development of nature-derived photocrosslinkable biomaterials for 3D printing in tissue engineering. Biomater. Res. 2019, 23, 18. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Suma, T.; Richardson, J.J.; Ejima, H. Modular Assembly of Biomaterials Using Polyphenols as Building Blocks. ACS Biomater. Sci. Eng. 2019, 5, 5578–5596. [Google Scholar] [CrossRef]
- Riemann, A.; Wußling, H.; Loppnow, H.; Fu, H.; Reime, S.; Thews, O. Acidosis differently modulates the inflammatory program in monocytes and macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Rajamäki, K.; Nordström, T.; Nurmi, K.; Åkerman, K.E.O.; Kovanen, P.T.; Öörni, K.; Eklund, K.K. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 2013, 288, 13410–13419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Song, M.; Li, J. Science review: Extracellular acidosis and the immune response: Clinical and physiologic implications. Crit. Care 2004, 8, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simionescu, D.; Casco, M.; Turner, J.; Rierson, N.; Yue, J.; Ning, K. Chemical stabilization of the extracellular matrix attenuates growth of experimentally induced abdominal aorta aneurysms in a large animal model. JVS Vasc. Sci. 2020, 1, 69–80. [Google Scholar] [CrossRef]
- Iahnke, A.O.E.S.; Stoll, L.; Bellé, A.S.; Hertz, P.F.; de Rios, A.O.; Rahier, H.; Flôres, S.H. Gelatin capsule residue-based films crosslinked with the natural agent genipin. Packag. Technol. Sci. 2020, 33, 15–26. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications. Catalysts 2019, 9, 1035. [Google Scholar] [CrossRef] [Green Version]
- Focaroli, S.; Teti, G.; Salvatore, V.; Durante, S.; Belmonte, M.M.; Giardino, R.; Mazzotti, A.; Bigi, A.; Falconi, M. Chondrogenic differentiation of human adipose mesenchimal stem cells: Influence of a biomimetic gelatin genipin crosslinked porous scaffold. Microsc. Res. Tech. 2014, 77, 928–934. [Google Scholar] [CrossRef]
- Gattazzo, F.; De Maria, C.; Rimessi, A.; Donà, S.; Braghetta, P.; Pinton, P.; Vozzi, G.; Bonaldo, P. Gelatin-genipin-based biomaterials for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2763–2777. [Google Scholar] [CrossRef]
- Koo, H.-J.; Song, Y.S.; Kim, H.-J.; Lee, Y.-H.; Hong, S.-M.; Kim, S.-J.; Kim, B.-C.; Jin, C.; Lim, C.-J.; Park, E.-H. Antiinflammatory effects of genipin, an active principle of gardenia. Eur. J. Pharmacol. 2004, 495, 201–208. [Google Scholar] [CrossRef]
- Kucharska, A.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [Green Version]
- West, B.J.; Uwaya, A.; Isami, F.; Deng, S.; Nakajima, S.; Jensen, C.J. Antiglycation Activity of Iridoids and Their Food Sources. Int. J. Food Sci. 2014, 2014, 276950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borzenkov, M.; Chirico, G.; Collini, M.; Pallavicini, P. Gold Nanoparticles for Tissue Engineering. In Environmental Nanotechnology. Environmental Chemistry for A Sustainable World; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 14, pp. 343–390. [Google Scholar]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, R.K.; Thennarasu, S.; Mandal, A.B. Resveratrol stabilized gold nanoparticles enable surface loading of doxorubicin and anticancer activity. Colloids Surf. B Biointerfaces 2014, 114, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, C.; He, Q.; Liao, X.; Shi, B. Collagen fiber with surface-grafted polyphenol as a novel support for Pd(0) nanoparticles: Synthesis, characterization and catalytic application. Mater. Sci. Eng. C 2010, 30, 770–776. [Google Scholar] [CrossRef]
- Navarro, J.; Calderon, G.A.; Miller, J.S.; Fisher, J.P. Bioinks for Three-Dimensional Printing in Regenerative Medicine. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 805–830. [Google Scholar] [CrossRef]
- Amini, S.; Saudi, A.; Amirpour, N.; Jahromi, M.; Najafabadi, S.S.; Kazemi, M.; Rafienia, M.; Salehi, H. Application of electrospun polycaprolactone fibers embedding lignin nanoparticle for peripheral nerve regeneration: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 159, 154–173. [Google Scholar] [CrossRef]
- Farcal, L.; Torres Andón, F.; Di Cristo, L.; Rotoli, B.M.; Bussolati, O.; Bergamaschi, E.; Mech, A.; Hartmann, N.B.; Rasmussen, K.; Riego-Sintes, J.; et al. Comprehensive In Vitro Toxicity Testing of a Panel of Representative Oxide Nanomaterials: First Steps towards an Intelligent Testing Strategy. PLoS ONE 2015, 10, e0127174. [Google Scholar] [CrossRef]
- Mitov, M.I.; Patil, V.S.; Alstott, M.C.; Dziubla, T.; Butterfield, D.A. In Vitro Cellular Assays for Oxidative Stress and Biomaterial Response. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 145–186. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; de Cienfuegos, G.A.; de Pablo, M.A. Dietary Antioxidants: Immunity and Host Defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Choksi, S.; Chen, K.; Pobezinskaya, Y.; Linnoila, I.; Liu, Z.-G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013, 23, 898–914. [Google Scholar] [CrossRef] [Green Version]
- López-Varela, S.; González-Gross, M.; Marcos, A. Functional foods and the immune system: A review. Eur. J. Clin. Nutr. 2002, 56, S29–S33. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Aladedunye, F.; Rodriguez-Juarez, R.; Li, D.; Thomas, J.; Kovalchuk, O.; Przybylski, R. Novel antioxidants are not toxic to normal tissues but effectively kill cancer cells. Cancer Biol. Ther. 2013, 14, 907–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokorný, J. Are natural antioxidants better and safer than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Rubio-Elizalde, I.; Bernáldez-Sarabia, J.; Moreno-Ulloa, A.; Vilanova, C.; Juárez, P.; Licea-Navarro, A.; Castro-Ceseña, A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 2019, 206, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017, 227, 9–15. [Google Scholar] [CrossRef]
- Arriagada, F.; Günther, G.; Nos, J.; Nonell, S.; Olea-Azar, C.; Morales, J. Antioxidant Nanomaterial Based on Core–Shell Silica Nanospheres with Surface-Bound Caffeic Acid: A Promising Vehicle for Oxidation-Sensitive Drugs. Nanomaterials 2019, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Shi, Z.; Neoh, K.G.; Kang, E.T. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jin, H.; Xiao, J.; Yin, X.; Liu, X.; Li, D.; Huang, Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018, 111, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Barrino, F.; Poggetto, G.D.; Crescente, G.; Piccolella, S.; Pacifico, S. Chlorogenic acid entrapped in hybrid materials with high PEG content: A strategy to obtain antioxidant functionalized biomaterials? Materials 2019, 12, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraipandy, N.; Kiran, M.S.; Fathima, N.N. Anti-oxidant enriched hybrid nanofibers: Effect on mechanical stability and biocompatibility. Int. J. Biol. Macromol. 2018, 117, 209–217. [Google Scholar] [CrossRef]
- Kang, B.; Vales, T.; Cho, B.-K.; Kim, J.-K.; Kim, H.-J. Development of Gallic Acid-Modified Hydrogels Using Interpenetrating Chitosan Network and Evaluation of Their Antioxidant Activity. Molecules 2017, 22, 1976. [Google Scholar] [CrossRef] [Green Version]
- Panzella, L.; Cerruti, P.; Ambrogi, V.; Agustin-Salazar, S.; D’Errico, G.; Carfagna, C.; Goya, L.; Ramos, S.; Martín, M.A.; Napolitano, A.; et al. A Superior All-Natural Antioxidant Biomaterial from Spent Coffee Grounds for Polymer Stabilization, Cell Protection, and Food Lipid Preservation. ACS Sustain. Chem. Eng. 2016, 4, 1169–1179. [Google Scholar] [CrossRef]
- Mishra, D.; Khare, P.; Singh, D.K.; Luqman, S.; Ajaya Kumar, P.V.; Yadav, A.; Das, T.; Saikia, B.K. Retention of antibacterial and antioxidant properties of lemongrass oil loaded on cellulose nanofibre-poly ethylene glycol composite. Ind. Crops Prod. 2018, 114, 68–80. [Google Scholar] [CrossRef]
- Guamán-Balcázar, M.C.; Montes, A.; Pereyra, C.; Martínez de la Ossa, E. Production of submicron particles of the antioxidants of mango leaves/PVP by supercritical antisolvent extraction process. J. Supercrit. Fluids 2019, 143, 294–304. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Nune, M.; Manchineella, S.; Govindaraju, T.; Narayan, K.S. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater. Sci. Eng. C 2019, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite–trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.A.; Ly, K.L.; Fox, K.E.; Tran, P.A.; Nguyen, T.H. Immobilization of antimicrobial silver and antioxidant flavonoid as a coating for wound dressing materials. Int. J. Nanomed. 2019, 14, 9929–9939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arriagada, F.; Günther, G.; Morales, J. Nanoantioxidant–Based Silica Particles as Flavonoid Carrier for Drug Delivery Applications. Pharmaceutics 2020, 12, 302. [Google Scholar] [CrossRef] [Green Version]
- Darie-Niţă, R.N.; Vasile, C.; Stoleru, E.; Pamfil, D.; Zaharescu, T.; Tarţău, L.; Tudorachi, N.; Brebu, M.A.; Pricope, G.M.; Dumitriu, R.P.; et al. Evaluation of the rosemary extract effect on the properties of polylactic acid-based materials. Materials 2018, 11, 1825. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liu, J.; Luo, X.; Xiong, P.; Gao, S.; Yan, J.; Li, Y.; Cheng, Y.; Xi, T. A tannic acid-modified fluoride pre-treated Mg–Zn–Y–Nd alloy with antioxidant and platelet-repellent functionalities for vascular stent application. J. Mater. Chem. B 2019, 7, 7314–7325. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Hwang, C.H.; Kim, H.E.; Jeong, S.H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018, 186, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Bardania, H.; Mahmoudi, R.; Bagheri, H.; Salehpour, Z.; Fouani, M.H.; Darabian, B.; Khoramrooz, S.S.; Mousavizadeh, A.; Kowsari, M.; Moosavifard, S.E.; et al. Facile preparation of a novel biogenic silver-loaded Nanofilm with intrinsic anti-bacterial and oxidant scavenging activities for wound healing. Sci. Rep. 2020, 10, 6129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for osral local delivery. Mater. Sci. Eng. C 2017, 81, 588–596. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Wadhwa, P.; Hong, J.W.; Hong, Y.G.; Jeon, J.M.; Lee, E.S.; Yang, Y.H. Lipase mediated functionalization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with ascorbic acid into an antioxidant active biomaterial. Int. J. Biol. Macromol. 2019, 123, 117–123. [Google Scholar] [CrossRef]
- Fu, S.-Z.; Meng, X.-H.; Fan, J.; Yang, L.-L.; Wen, Q.-L.; Ye, S.-J.; Lin, S.; Wang, B.-Q.; Chen, L.-L.; Wu, J.-B.; et al. Acceleration of dermal wound healing by using electrospun curcumin-loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) fibrous mats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 533–542. [Google Scholar] [CrossRef]
- Wu, X.; Dai, H.; Xu, C.; Liu, L.; Li, S. Citric acid modification of a polymer exhibits antioxidant and anti-inflammatory properties in stem cells and tissues. J. Biomed. Mater. Res. Part A 2019, 107, 2414–2424. [Google Scholar] [CrossRef]
- Wattamwar, P.P.; Biswal, D.; Cochran, D.B.; Lyvers, A.C.; Eitel, R.E.; Anderson, K.W.; Hilt, J.Z.; Dziubla, T.D. Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012, 8, 2529–2537. [Google Scholar] [CrossRef]
- Luisa, D.P.-A.M.; Griselda, R.-M.; Valentín, M.-L.; Carmina, O.-S.; Cristina, V.-M.; JJ, M.; Maykel, G.-T.; David, Q.-G.; Roberto, S.-S.; Gerardo, L.-G. Curcumin-loaded poly-ε-caprolactone nanoparticles show antioxidant and cytoprotective effects in the presence of reactive oxygen species. J. Bioact. Compat. Polym. 2020, 35, 270–285. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, S.K.; Je, J.Y. Chitosan gallate as potential antioxidant biomaterial. Bioorg. Med. Chem. Lett. 2011, 21, 3070–3073. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Yang, I.H.; Deen, G.R.; Mo, X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surfaces B Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Tabrizi, M.H.; Moghaddam, M.N.; Shahraki, F.; Yadamani, S. Rapeseed flower pollen bio-green synthesized silver nanoparticles: A promising antioxidant, anticancer and antiangiogenic compound. JBIC J. Biol. Inorg. Chem. 2019, 24, 395–404. [Google Scholar] [CrossRef]
- Shiekh, P.A.; Singh, A.; Kumar, A. Engineering Bioinspired Antioxidant Materials Promoting Cardiomyocyte Functionality and Maturation for Tissue Engineering Application. ACS Appl. Mater. Interfaces 2018, 10, 3260–3273. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, B.; Sun, Y.; Wang, C.; Mukherjee, S.; Yang, C.; Chen, Y. Injectable Enzyme-Based Hydrogel Matrix with Precisely Oxidative Stress Defense for Promoting Dermal Repair of Burn Wound. Macromol. Biosci. 2020, 20, 2000036. [Google Scholar] [CrossRef]
- Haghparasti, Z.; Mahdavi Shahri, M. Green synthesis of water-soluble nontoxic inorganic polymer nanocomposites containing silver nanoparticles using white tea extract and assessment of their in vitro antioxidant and cytotoxicity activities. Mater. Sci. Eng. C 2018, 87, 139–148. [Google Scholar] [CrossRef]
- Suganya, S.; Venugopal, J.; Ramakrishna, S.; Lakshmi, B.S.; Dev, V.R.G. Naturally derived biofunctional nanofibrous scaffold for skin tissue regeneration. Int. J. Biol. Macromol. 2014, 68, 135–143. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.; Kim, B.S.; An, Y.H.; Lee, U.J.; Lee, S.H.; Kim, S.L.; Kim, B.G.; Hwang, N.S. Fabrication of polyphenol-incorporated anti-inflammatory hydrogel via high-affinity enzymatic crosslinking for wet tissue adhesion. Biomaterials 2020, 242, 119905. [Google Scholar] [CrossRef]
- Perut, F.; Montufar, E.B.; Ciapetti, G.; Santin, M.; Salvage, J.; Traykova, T.; Planell, J.A.; Ginebra, M.P.; Baldini, N. Novel soybean/gelatine-based bioactive and injectable hydroxyapatite foam: Material properties and cell response. Acta Biomater. 2011, 7, 1780–1787. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Ramalingam, R.; Dhand, C.; Lakshminarayanan, R.; Sadiq, A.; Gandhimathi, C.; Ramakrishna, S.; Bay, B.H.; Venugopal, J.R.; Srinivasan, D.K. Biocompatible aloe vera and tetracycline hydrochloride loaded hybrid nanofibrous scaffolds for skin tissue engineering. Int. J. Mol. Sci. 2019, 20, 5174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Cifuentes, L.; Jiménez, R.A.; Fontanilla, M.R. Evaluation of collagen type I scaffolds including gelatin-collagen microparticles and Aloe vera in a model of full-thickness skin wound. Drug Deliv. Transl. Res. 2019, 9, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.W.; Ko, S.C.; Je, J.Y.; Kim, Y.M.; Oh, J.H.; Jung, W.K. Fabrication, characterization and determination of biological activities of poly(ε-caprolactone)/chitosan-caffeic acid composite fibrous mat for wound dressing application. Int. J. Biol. Macromol. 2016, 93, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan-caffeic acid-genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Millán, D.; Jiménez, R.A.; Nieto, L.E.; Linero, I.; Laverde, M.; Fontanilla, M.R. Preclinical evaluation of collagen type I scaffolds, including gelatin-collagen microparticles and loaded with a hydroglycolic Calendula officinalis extract in a lagomorph model of full-thickness skin wound. Drug Deliv. Transl. Res. 2016, 6, 57–66. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, R.; Lan, W.; Qin, W. Fabrication of Electrospun Polylactic Acid/Cinnamaldehyde/β-Cyclodextrin Fibers as an Antimicrobial Wound Dressing. Polymers 2017, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Liguori, A.; Uranga, J.; Panzavolta, S.; Guerrero, P.; de la Caba, K.; Focarete, M.L. Electrospinning of fish gelatin solution containing citric acid: An environmentally friendly approach to prepare crosslinked gelatin fibers. Materials 2019, 12, 2808. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Q.; Liu, Y.; Ju, F.; Ma, Q.; He, Q. In vivo evaluation of enhanced drug carrier efficiency and cardiac anti-hypertrophy therapeutic potential of nano-curcumin encapsulated photo-plasmonic nanoparticles combined polymerized nano-vesicles: A novel strategy. J. Photochem. Photobiol. B Biol. 2019, 199, 111619. [Google Scholar] [CrossRef]
- Huang, Z.; Delparastan, P.; Burch, P.; Cheng, J.; Cao, Y.; Messersmith, P.B. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci. 2018, 6, 2487–2495. [Google Scholar] [CrossRef] [Green Version]
- Khakestani, M.; Jafari, S.H.; Zahedi, P.; Bagheri, R.; Hajiaghaee, R. Physical, morphological, and biological studies on PLA/nHA composite nanofibrous webs containing Equisetum arvense herbal extract for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45343. [Google Scholar] [CrossRef]
- Russo, N.; Cassinelli, C.; Torre, E.; Morra, M.; Iviglia, G. Improvement of the Physical Properties of Guided Bone Regeneration Membrane from Porcine Pericardium by Polyphenols-Rich Pomace Extract. Materials 2019, 12, 2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandhasamy, S.; Perumal, S.; Madhan, B.; Umamaheswari, N.; Banday, J.A.; Perumal, P.T.; Santhanakrishnan, V.P. Synthesis and Fabrication of Collagen-Coated Ostholamide Electrospun Nanofiber Scaffold for Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 8556–8568. [Google Scholar] [CrossRef] [PubMed]
- Duraipandy, N.; Lakra, R.; Vinjimur Srivatsan, K.; Ramamoorthy, U.; Korrapati, P.S.; Kiran, M.S. Plumbagin caged silver nanoparticle stabilized collagen scaffold for wound dressing. J. Mater. Chem. B 2015, 3, 1415–1425. [Google Scholar] [CrossRef]
- Sheu, S.Y.; Chen, W.S.; Sun, J.S.; Lin, F.H.; Wu, T. Biological characterization of oxidized hyaluronic acid/resveratrol hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Allahyari, Z.; Salehi, M. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Maya Nandkumar, A.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 346. [Google Scholar] [CrossRef] [Green Version]
- Renò, F.; Aina, V.; Gatti, S.; Cannas, M. Effect of vitamin E addition to poly(d,l)-lactic acid on surface properties and osteoblast behaviour. Biomaterials 2005, 26, 5594–5599. [Google Scholar] [CrossRef]
- Dias, F.T.G.; Ingracio, A.R.; Nicoletti, N.F.; Menezes, F.C.; Dall Agnol, L.; Marinowic, D.R.; Soares, R.M.D.; da Costa, J.C.; Falavigna, A.; Bianchi, O. Soybean-modified polyamide-6 mats as a long-term cutaneous wound covering. Mater. Sci. Eng. C 2019, 99, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Trifković, K.T.; Milašinović, N.Z.; Djordjević, V.B.; Krušić, M.T.K.; Knežević-Jugović, Z.D.; Nedović, V.A.; Bugarski, B.M. Chitosan microbeads for encapsulation of thyme (Thymus serpyllum L.) polyphenols. Carbohydr. Polym. 2014, 111, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, N.T.; Khorram, M.; Zomorodia, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-F.; Chen, Y.-C.; Chen, R.-N.; Chen, L.-C.; Ho, H.-O.; Tsung, Y.-H.; Sheu, M.-T.; Liu, D.-Z. Improving the Stability of Astaxanthin by Microencapsulation in Calcium Alginate Beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Z.; Sun, X.; Zhang, S.; Wang, S.; Feng, F.; Wang, D.; Xu, Y. Fabrication and Characterization of β-Lactoglobulin-Based Nanocomplexes Composed of Chitosan Oligosaccharides as Vehicles for Delivery of Astaxanthin. J. Agric. Food Chem. 2018, 66, 6717–6726. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Bastias Venegas, J.; Pauthe, E.; et al. Astaxanthin-Loaded Nanostructured Lipid Carriers for Preservation of Antioxidant Activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef] [Green Version]
- Tyliszczak, B.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Bogucki, R.; Olejnik, E.; Kinasiewicz, J.; Głąb, M. Hydrogels containing caffeine and based on Beetosan®—Proecological chitosan—Preparation, characterization, and in vitro cytotoxicity. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 931–935. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Jain, S.; Meka, S.R.K.; Chatterjee, K. Curcumin eluting nanofibers augment osteogenesis toward phytochemical based bone tissue engineering. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef]
- Gupta, P.; Jordan, C.T.; Mitov, M.I.; Butterfield, D.A.; Hilt, J.Z.; Dziubla, T.D. Controlled curcumin release via conjugation into PBAE nanogels enhances mitochondrial protection against oxidative stress. Int. J. Pharm. 2016, 511, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Vanat, P.; Marques-da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Metal alginates for polyphenol delivery systems: Studies on crosslinking ions and easy-to-use patches for release of protective flavonoids in skin. Bioact. Mater. 2020, 5, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural extracts into chitosan nanocarriers for rosmarinic acid drug delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodadová, A.; Vitková, Z.; Herdová, P.; Ťažký, A.; Oremusová, J.; Grančai, D.; Mikuš, P. Formulation of sage essential oil (Salvia officinalis, L.) monoterpenes into chitosan hydrogels and permeation study with GC-MS analysis. Drug Dev. Ind. Pharm. 2015, 41, 1080–1088. [Google Scholar] [CrossRef]
- Amariei, G.; Boltes, K.; Letón, P.; Iriepa, I.; Moraleda, I.; Rosal, R. Poly (amidoamine) dendrimers grafted on electrospun poly (acrylic acid)/poly (vinyl alcohol) membranes for host–guest encapsulation of antioxidant thymol. J. Mater. Chem. B 2017, 5, 6776–6785. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, S556–S566. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Garcia-Garcia, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Delgado, A.; Igartua, M. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int. J. Pharm. 2019, 556, 320–329. [Google Scholar] [CrossRef]
- Silva, S.S.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Thompson, Z.; Rahman, S.; Yarmolenko, S.; Sankar, J.; Kumar, D.; Bhattarai, N. Fabrication and Characterization of Magnesium Ferrite-Based PCL/Aloe Vera Nanofibers. Materials 2017, 10, 937. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Composite wound dressings of pectin and gelatin with aloe vera and curcumin as bioactive agents. Int. J. Biol. Macromol. 2016, 82, 104–113. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core–Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly (lactic- co-glycolic acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, J.; Guan, L.; Zhang, Y.; Dong, P.; Li, J.; Liang, X.; Komiyama, M. Fabrication of aqueous nanodispersion from natural DNA and chitosan as eminent carriers for water-insoluble bioactives. Int. J. Biol. Macromol. 2018, 118, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Gregnanin, G.; Cencetti, C.; Di Meo, C.; Gueguen, V.; Letourneur, D.; Meddahi-Pellé, A.; Pavon-Djavid, G.; Matricardi, P. PVA/Dextran hydrogel patches as delivery system of antioxidant astaxanthin: A cardiovascular approach. Biomed. Mater. 2018, 13. [Google Scholar] [CrossRef]
- Vargas, E.A.T.; do Vale Baracho, N.C.; de Brito, J.; de Queiroz, A.A.A. Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta Biomater. 2010, 6, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Preparation and characterization of Calendula officinalis-loaded PCL/gum arabic nanocomposite scaffolds for wound healing applications. Iran. Polym. J. 2019, 28, 51–63. [Google Scholar] [CrossRef]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, L.; Song, L.L.; Chen, S.; Gao, Y. α-Tocopherol liposome loaded chitosan hydrogel to suppress oxidative stress injury in cardiomyocytes. Int. J. Biol. Macromol. 2019, 125, 1192–1202. [Google Scholar] [CrossRef]
- Kheradvar, S.A.; Nourmohammadi, J.; Tabesh, H.; Bagheri, B. Starch nanoparticle as a vitamin E-TPGS carrier loaded in silk fibroin-poly (vinyl alcohol)-Aloe vera nanofibrous dressing. Colloids Surfaces B Biointerfaces 2018, 166, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Islam, R.; Rana, M.M.; Spitzhorn, L.S.; Rahman, M.S.; Adjaye, J.; Asaduzzaman, S.M. Characterization of burn wound healing gel prepared from human amniotic membrane and Aloe vera extract. BMC Complement. Altern. Med. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goonoo, N.; Fahmi, A.; Jonas, U.; Gimié, F.; Arsa, I.A.; Bénard, S.; Schönherr, H.; Bhaw-Luximon, A. Improved Multicellular Response, Biomimetic Mineralization, Angiogenesis, and Reduced Foreign Body Response of Modified Polydioxanone Scaffolds for Skeletal Tissue Regeneration. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; Del Valle, L.J.; Díaz, A.; Casas, M.T.; Puiggalí, J. Polylactide nanofibers loaded with vitamin B6 and polyphenols as bioactive platform for tissue engineering. Macromol. Res. 2013, 21, 775–787. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Rubini, K.; Fini, M.; Bigi, A. Antioxidant and bone repair properties of quercetin-functionalized hydroxyapatite: An in vitro osteoblast-osteoclast-endothelial cell co-culture study. Acta Biomater. 2016, 32, 298–308. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Rubini, K.; Fini, M.; Bigi, A. Quercetin and alendronate multi-functionalized materials as tools to hinder oxidative stress damage. J. Biomed. Mater. Res. Part A 2017, 105, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. Strategic design of cardiac mimetic core-shell nanofibrous scaffold impregnated with Salvianolic acid B and Magnesium L-ascorbic acid 2 phosphate for myoblast differentiation. Mater. Sci. Eng. C 2018, 90, 131–147. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Shapouri Moghadam, A.; Enderami, S.E.; Islami, M.; Kaabi, M.; Saburi, E.; Daei Farshchi, A.; Soleimanifar, F.; Mansouri, V. Aloe Vera—Derived Gel-Blended Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanofibrous Scaffold for Bone Tissue Engineering. ASAIO J. 2019. [Google Scholar] [CrossRef]
- Bhaarathy, V.; Venugopal, J.; Gandhimathi, C.; Ponpandian, N.; Mangalaraj, D.; Ramakrishna, S. Biologically improved nanofibrous scaffolds for cardiac tissue engineering. Mater. Sci. Eng. C 2014, 44, 268–277. [Google Scholar] [CrossRef]
- Choi, B.Y.; Chalisserry, E.P.; Kim, M.H.; Kang, H.W.; Choi, I.W.; Nam, S.Y. The influence of astaxanthin on the proliferation of adipose-derived mesenchymal stem cells in gelatin-methacryloyl (GelMA) hydrogels. Materials 2019, 12, 2416. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, D.; Qiao, R.; Dao, J.; Su, J.; Jiang, C.; Wang, X.; Gao, M.; Zhong, J. Soybean Lecithin-Mediated Nanoporous PLGA Microspheres with Highly Entrapped and Controlled Released BMP-2 as a Stem Cell Platform. Small 2018, 14, e1800063. [Google Scholar] [CrossRef]
- Aghamohamadi, N.; Sanjani, N.S.; Majidi, R.F.; Nasrollahi, S.A. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater. Sci. Eng. C 2019, 94, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Yang, F.; Wang, T.; Ni, M.; Chen, Y.; Yang, F.; Huang, D.; Fu, C.; Wang, S. Preparation and Characterization of Chitosan-Based Ternary Blend Edible Films with Efficient Antimicrobial Activities for Food Packaging Applications. J. Food Sci. 2019, 84, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial Carvacrol Incorporated in Flaxseed Gum-Sodium Alginate Active Films to Improve the Quality Attributes of Chinese Sea bass (Lateolabrax maculatus) during Cold Storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [Green Version]
- Kesici Güler, H.; Cengiz Çallıoğlu, F.; Sesli Çetin, E. Antibacterial PVP/cinnamon essential oil nanofibers by emulsion electrospinning. J. Text. Inst. 2019, 110, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of poly (Lactic acid)/chitosan fibers loaded with essential oil for antimicrobial applications. Nanomaterials 2017, 7, 194. [Google Scholar] [CrossRef] [Green Version]
- Sundeep, D.; Vijaya Kumar, T.; Rao, P.S.S.; Ravikumar, R.V.S.S.N.; Gopala Krishna, A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog. Biomater. 2017, 6, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Ragab, T.I.M.; Nada, A.A.; Ali, E.A.; Shalaby, A.S.G.; Soliman, A.A.F.; Emam, M.; El Raey, M.A. Soft hydrogel based on modified chitosan containing P. granatum peel extract and its nano-forms: Multiparticulate study on chronic wounds treatment. Int. J. Biol. Macromol. 2019, 135, 407–421. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Li, D.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Cao, T.L.; Yang, S.Y.; Song, K. Bin Development of burdock root inulin/chitosan blend films containing oregano and thyme essential oils. Int. J. Mol. Sci. 2018, 19, 131. [Google Scholar] [CrossRef] [Green Version]
- Vafania, B.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of thyme essential oil in chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to reduce nitrite in sausages. Food Bioprod. Process. 2019, 116, 240–248. [Google Scholar] [CrossRef]
- Wang, T.; Luo, Y. Chitosan Hydrogel Beads Functionalized with Thymol-Loaded Solid Lipid–Polymer Hybrid Nanoparticles. Int. J. Mol. Sci. 2018, 19, 3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of cellulose acetate for controlled release of thymol. Carbohydr. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Visai, L.; Renò, F.; Cangini, I.; Rizzi, M.; Poggi, A.; Montanaro, L.; Rimondini, L.; Arciola, C.R. Bacterial adhesion to poly-(D,L) lactic acid blended with vitamin E: Toward gentle anti-infective biomaterials. J. Biomed. Mater. Res. Part A 2015, 103, 1447–1458. [Google Scholar] [CrossRef]
- Paul, K.; Darzi, S.; McPhee, G.; Del Borgo, M.P.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019, 97, 162–176. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A.; Moshiri, A. Healing potential of injectable Aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res. 2019, 377, 215–227. [Google Scholar] [CrossRef]
- Giménez-Siurana, A.; Gómez García, F.; Pagan Bernabeu, A.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; López-Jornet, P. Chemoprevention of Experimental Periodontitis in Diabetic Rats with Silk Fibroin Nanoparticles Loaded with Resveratrol. Antioxidants 2020, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Pérez, A.A.; Rodriguez-Nogales, A.; Ortiz-Cullera, V.; Algieri, F.; Zorrilla, P.; Rodriguez-Cabezas, M.E.; Garrido-Mesa, N.; Utrilla, M.P.; Matteis, L.; Garrido-Mesa, J.; et al. Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int. J. Nanomed. 2014, 9, 4507. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.C.; Marques, A.P.; Silva, S.S.; Oliveira, J.M.; Mano, J.F.; Castro, A.G.; Reis, R.L. In vitro evaluation of the behaviour of human polymorphonuclear neutrophils in direct contact with chitosan-based membranes. J. Biotechnol. 2007, 132, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, T.C.; Marques, A.P.; Silva, S.S.; Oliveira, J.M.; Mano, J.F.; Castro, A.G.; van Griensven, M.; Reis, R.L. Chitosan Improves the Biological Performance of Soy-Based Biomaterials. Tissue Eng. Part A 2010, 16, 2883–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sigaroodi, F.; Shafaei, H.; Karimipour, M.; Dolatkhah, M.A.; Delazar, A. Aloe Vera/Collagen Mixture Induces Integrin α1β1 and PECAM-1Genes Expression in Human Adipose-Derived Stem Cells. Adv. Pharm. Bull. 2019, 9, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; de Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1938–1949. [Google Scholar] [CrossRef]
- Pires, A.L.R.; de Azevedo Motta, L.; Dias, A.M.A.; de Sousa, H.C.; Moraes, Â.M.; Braga, M.E.M. Towards wound dressings with improved properties: Effects of poly (dimethylsiloxane) on chitosan-alginate films loaded with thymol and beta-carotene. Mater. Sci. Eng. C 2018, 93, 595–605. [Google Scholar] [CrossRef]
- Gao, X.; Qin, W.; Wang, P.; Wang, L.; Weir, M.; Reynolds, M.; Zhao, L.; Lin, Z.; Xu, H. Nano-Structured Demineralized Human Dentin Matrix to Enhance Bone and Dental Repair and Regeneration. Appl. Sci. 2019, 9, 1013. [Google Scholar] [CrossRef] [Green Version]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering. Bone Joint Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, M.; Ferraris, S.; Prenesti, E.; Casalegno, V.; Spriano, S. Grafting of Gallic Acid onto a Bioactive Ti6Al4V Alloy: A Physico-Chemical Characterization. Coatings 2019, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Song, J.E.; Tripathy, N.; Lee, D.H.; Park, J.H.; Khang, G. Quercetin Inlaid Silk Fibroin/Hydroxyapatite Scaffold Promotes Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 32955–32964. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Goeckelmann, M.J.; Niclas, A.A.; Montufar, E.B.; Ginebra, M.-P.; Planell, J.A.; Santin, M.; Ignatius, A. In vivo performance of novel soybean/gelatin-based bioactive and injectable hydroxyapatite foams. Acta Biomater. 2015, 12, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tığlı Aydın, R.S.; Hazer, B.; Acar, M.; Gümüşderelioğlu, M. Osteogenic activities of polymeric soybean oil-g-polystyrene membranes. Polym. Bull. 2013, 70, 2065–2082. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, J.I.; Hwang, T.I.; Sim, B.R.; Khang, G. Bioengineered osteoinductive broussonetia kazinoki/silk fibroin composite scaffolds for bone tissue regeneration. ACS Appl. Mater. Interfaces 2017, 9, 1384–1394. [Google Scholar] [CrossRef]
- Dhanasekaran, S. Phytochemical characteristics of aerial part of Cissus quadrangularis (L) and its in-vitro inhibitory activity against leukemic cells and antioxidant properties. Saudi J. Biol. Sci. 2020, 27, 1302–1309. [Google Scholar] [CrossRef]
- Murthy, K.N.C.; Vanitha, A.; Mahadeva Swamy, M.; Ravishankar, G.A. Antioxidant and Antimicrobial Activity of Cissus quadrangularis L. J. Med. Food 2003, 6, 99–105. [Google Scholar] [CrossRef]
- Tamburaci, S.; Kimna, C.; Tihminlioglu, F. Novel phytochemical Cissus quadrangularis extract–loaded chitosan/Na-carboxymethyl cellulose–based scaffolds for bone regeneration. J. Bioact. Compat. Polym. 2018, 33, 629–646. [Google Scholar] [CrossRef]
- Renò, F.; Traina, V.; Cannas, M. Cellular behavior of neointima-like cells onto Vitamin E-enriched poly(d,l) lactic acid. Biomol. Eng. 2007, 24, 307–312. [Google Scholar] [CrossRef]
- Vergalito, F.; Pietrangelo, L.; Petronio Petronio, G.; Colitto, F.; Alfio Cutuli, M.; Magnifico, I.; Venditti, N.; Guerra, G.; Di Marco, R. Vitamin E for prevention of biofilm-caused Healthcare-associated infections. Open Med. 2019, 15, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims-Robinson, C.; Hur, J.; Hayes, J.M.; Dauch, J.R.; Keller, P.J.; Brooks, S.V.; Feldman, E.L. The Role of Oxidative Stress in Nervous System Aging. PLoS ONE 2013, 8, e68011. [Google Scholar] [CrossRef] [Green Version]
- Sensharma, P.; Madhumathi, G.; Jayant, R.D.; Jaiswal, A.K. Biomaterials and cells for neural tissue engineering: Current choices. Mater. Sci. Eng. C 2017, 77, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; Vicanova, J.; Cramers, P.; Vrolijk, H.; Pavel, S. The Combined Effects of Extracts Containing Carotenoids and Vitamins E and C on Growth and Pigmentation of Cultured Human Melanocytes. Skin Pharmacol. Physiol. 2004, 17, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [Green Version]
- Marrazzo, P.; Crupi, A.N.; Alviano, F.; Teodori, L.; Bonsi, L. Exploring the roles of MSCs in infections: Focus on bacterial diseases. J. Mol. Med. 2019. [Google Scholar] [CrossRef]
- Ajith, Y.; Dimri, U.; Dixit, S.K.; Singh, S.K.; Gopalakrishnan, A.; Madhesh, E.; Rajesh, J.B.; Sangeetha, S.G. Immunomodulatory basis of antioxidant therapy and its future prospects: An appraisal. Inflammopharmacology 2017, 25, 487–498. [Google Scholar] [CrossRef]






| Property | Antioxidant | Biomaterial (Scaffold) |
|---|---|---|
| Scavenging activity | Aloe vera (extract) [109] Astaxanthin [110] Caffeic acid [111] Carvacrol [112] Catechin [113] Chlorogenic acid [114] Cinnamon E.O. [115] Curcumin [116] Eugenol [112] Fenugreek (seed absolut) [117] Gallic acid [118] Hydrolyzed spent coffee grounds [119] Lemongrass E.O. [120] Mango (extract) [121,122] Melanin [123] Moringa oleifera (extract) [109] Quercetin [113,124,125] Rosmarinic acid [126] Rosemary (extract) [127] Tannic acid [128,129] Teucrium polium (extract) [130] Thyme (polyphenol extract) [131] Thymol [132] Vitamin C [133] | Cellulose-PEG [120] CH [112,113,122,132] CH-derivates [118,131] CH-gum [115] HA [129] Halloyste [124] Magnesium alloy [128] P(3HB-co-3HV) [133] PDMS [125] PE [119] PEG–ALG [109] PEG-PCL [134] PLA –PEG [130] PLA [127] PVP [121] SF -collagen [117] SF [123] Silica [111,126] Silica-PEG [114] |
| Indirect antioxidant effects (including cytoprotection) | Citric acid [135] Curcumin [136,137] Cystamine [73] Gallic acid [138] Hydrolyzed spent coffee grounds [119] Lignin (alkali) [139] Quercetin [136] Rapeseed flower (pollen extract) [140] Vitamin C [141] Vitamin E [142] White tea (leaves extract) [143] | CH [138] CH-derivates [116] Hyaluronic acid-β-cyclodextrin [142] Montmorillonite [143] PBAE [136] PCL [137,139] PE [119] PU [141] PVA [135] |
| Biodegradation modulation | Aloe vera [144] EGCG [145] Soybean (extract) [146] Tannic acid [129] Vitamin C [133] | Gelatin-Hap [146] HA [129,145] P(3HB-co-3HV) [133] SF [144] |
| Biomechanical stability | Aloe vera (extract) [147,148] Caffeic acid [149,150] Calendula Officinalis [151] Cinnamaldhyde [152] Citric acid [153] Curcumin [154] EGCG [155] Ellagic acid [155] Equisetum arvense (extract) [156] Grape (pomace) [157] Lignin (kraft) [100] Ostholamide [158] Plumbagin [159] Resveratrol [160] Soybean (extract) [146] Tannic acid [131,155] Vitamin C [133]. | CH-Starch [131] Collagen [159] Collagen type I–Gelatin [148,151] Gelatin [153] Gelatin derivates-collagen [158] Gelatin-Hap [146] HA [160] P(3HB-co-3HV) [133] PCL [100,147] PCL-CH [149,150] PEG derivatives [155] PLA–derivates [152] PLA-Hap [156] PLGA [154] Porcine pericardium (decellularized) [157] |
| Hydrophilicity and wettability/swelling | Aloe vera (extract) [48,109] Astaxanthin [161] Berberine [162] Cinnamaldhyde [152] Curcumin [163,164] Lignin (kraft) [100] Ostholamide [158] Peppermint E.O. [165] Plumbagin [159] Rosemary (extract) [127] Sesamol [166] Soybean (oil) [167] Thymus (polyphenol extract) [168] Zataria Multiflora E.O. [169] | Cellulose acetate-gelatin [162] CH [168] Collagen [159,161] Gelatin derivates-collagen [158] HPG [164] PA-6 [167] PCL [48,100,165] PCL-gum [163] PEG-ALG [109] PLA [127,166] PLA-derivatives [152] PVA derivates [169] |
| Controlled release | Astaxanthin [170,171,172] Caffeic acid [173] Curcumin [116,174,175,176] Cystamine [73] EGCG [177,178] Morin [126] Ostholamide [158] Quercetin [157] Rosmarinic acid [179] Sage E.O. [180] Thymol [181] Thymus (polyphenol extract) [168] | ALG [170,177] CH-derivates [116,171] CH [168,180] Gelatin [178] Gelatin derivates-collagen [158] PBAE [73,176] PCL [175] Porcine pericardium (decellularized) [157] PVA [174] PVA derivates [181] Silica [126] |
| Biocompatibility | Aloe Vera (extract) [182,183,184,185,186] Astaxanthin [187,188,189] Calendula Officinalis (extract) [190,191,192] Cinnamon, E.O. [193] Curcumin [116,134,154,163,164,186] Lemongrass E.O. [193] Peppermint E.O. [193] Plumbagin [159] Rosmarinic acid [194] Soybean (oil) [167] Tannic acid [195] Tyramine [145] Vitamin E [196] | Cellulose acetate [193] CH [184,194,196] CH-derivates [116] Collagen [159] Gelatin [186] HA [145] HPG [164,190] PA-6 [167] PCL [185] PCL-gum [163] PCL-gum [191,192] PEG-PCL [134] PLGA [154,182,183,187,188] Poly-tannic acid [195] PVA derivatives [189] |
| Cell proliferation | Aloe Vera (extract) [48,109,144,197,198,199] Caffeic acid [149,200] Curcumin [154] Lignin (alkali) [139] Moringa oleifera (extract) [109] p-coumaric acid [200] Quercetin [201,202] Salvianolic acid B [203] Thymol [132] Vitamin B6 [200] Vitamin C (Magnesium phosphate) [203] | Amnion (decellularized hydrogel) [198] CH [132] Hap [201,202] PCL [48,139] PCL-gelatin [203] PDX [199] PEG-ALG [109] PLA [200] PLGA [154] SF [144] SF-PVA [197] |
| Cell differentiation | Aloe vera (extract) [204,205] Astaxanthin [206] EGCG [178,207] Lignin (alkali) [139] (kraft) [100] Melanin [123] Quercetin [201] Salvianolic acid B [203] Soybean lecithin [208] Vitamin C (Magnesium phosphate) [203] Vitamin C [141] | Gelatin [178,207] Gelatin derivatives [206] Hap [201] PCL [100,139] PCL-gelatin [203] PHBV [204] PLA-PCL-SF [205] PLGA [208] PU [141] SF [123] |
| Antimicrobial activity | Aloe vera (extract) [209] Berberine [162] Caffeic acid [149] Calendula Officinalis (extract) [192,210] Carvacrol [112,211] Chlorogenic acid [114] Cinnamaldhyde [152] Cinnamon E.O. [193,212,213] Curcumin [174] Eugenol [112] Lemongrass E.O. [193] Mango (silver particles) [214] Moringa Oleifera (extract) [109] Ostholamide [158] Peppermint E.O. [165,193] Plumbagin [159] Punica granatum (peel extract) [215] Quercetin [125,201] Rosemary (extract) [127] Rosmarinic acid [216] Sage E.O. [180] Teucrium polium (extract) [130] Thyme and Oregano E.O. [217,218,219] Thymol [132,220,221] Vitamin E [222] Zataria Multiflora E.O. [169] | ALG [211] Cellulose acetate [193,221] Cellulose acetate-gelatin [162] Cellulose-PEG [120] CH [112,210,220] CH-derivatives [215,218] CH-gelatin [219] Collagen [159] Gelatin derivates-collagen [158] Gelatin-gum [216] Hap [201] Ionomer cement [214] PCL [165] PCL-CH [149] PCL-gum [191,192] PDMS [125] PEG-ALG [109] PLA [127] PLA-CH [212,213] PLA-derivatives [152,222] PLA-PEG [130] PVA [174] PVA derivates [169] PVP [209] Silica-PEG [114] β-cyclodextrin [217] |
| Immunomodulation and anti-inflammatory effect | Aloe vera (extract) [223,224] Citric acid [135] Curcumin [154] EGCG [145] Quercetin [202] Resveratrol [225,226] Soybean [227,228] | ALG [223] CH [227,228] Cow bone matrix (decellularized) [224] HA [145] Hap [202] PLGA [154] PVA [135] SF [225,226] |
| Therapeutic Application | Antioxidant | Biomaterial |
|---|---|---|
| Wound healing | Aloe vera [147,183,186,198,224,230] Astaxanthin [161] Berberine [162] Bixin [233] Calendula officinalis [190] Curcumin [134,147,164,186,230,233] Dopamine [125,142] EGCG [145] Moringa oleifera (extract) [109] Ostholamide [158] Quercetin [125] Sesamol [232] Soybean (extract) [167] Thymol [234] Tyramine [145] Vitamin E [142] β-Carotene [234] | ALG derivatives [109] Amnion (decellularized hydrogel) [198] Bone matrix (decellularized) [224] .Cellulose acetate–gelatin [162] Cellulose acetate derivatives [232] CH derivatives [116] CH-ALG [234] Collagen [161] Gelatin derivative [186] Gelatin derivatives-collagen [158] HA [145] HA-β-cyclodextrin [142] HPG [190] PA-6 [167] PCL [148,236] PDMS [125] PEG-PCL [134] PLA-HPG [164] PLGA derivatives [183] PMAA [230] |
| Bone defects | Aloe vera (acemannan/glucomannan) [199] (extract) [204] Broussonetia kazinoki (extract) [242] Cissus quadrangularis (extract) [245] Curcumin [175] EGCG [178,207] Equisetum arvense (extract) [156] Fenugreek (seed absolut) [117] Quercetin [204,205,243] Soybean (extract) [240] Soybean (oil) [241] | CH derivatives [245] Gelatin [178,207] Gelatin-Hap [240] Hap [201,202] PCL [175] PDX derivates [199] PHBV [204] PLA-Hap [156] PS [241] SF [242] SF-collagen [117] SF-Hap [239] |
| Cardiovascular diseases | Aloe Vera [205] Astaxanthin [189] Curcumin [154] Salvianolic acid B [203] Tannic acid [128] Vitamin C (Magnesium phosphate) [203] Vitamin E [196,246] | CH [196] Magnesium alloy [128] PCL-gelatin [203] PLA [246] PLA-PCL-SF [205] PLGA [154] PVA derivatives [189] |
| Neurological disorders | Lignin (alkali) [139] (kraft) ([100]) Melanin [123] | PCL [100,139] SF [123] |
| Other tissue disorders | Aloe vera [223] Resveratrol [160] | ALG [223] HA [160] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrazzo, P.; O’Leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. https://doi.org/10.3390/bioengineering7030104
Marrazzo P, O’Leary C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering. 2020; 7(3):104. https://doi.org/10.3390/bioengineering7030104
Chicago/Turabian StyleMarrazzo, Pasquale, and Cian O’Leary. 2020. "Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering" Bioengineering 7, no. 3: 104. https://doi.org/10.3390/bioengineering7030104
APA StyleMarrazzo, P., & O’Leary, C. (2020). Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering, 7(3), 104. https://doi.org/10.3390/bioengineering7030104