Encapsulation of Hydrophobic Drugs in Shell-by-Shell Coated Nanoparticles for Radio—and Chemotherapy—An In Vitro Study
Abstract
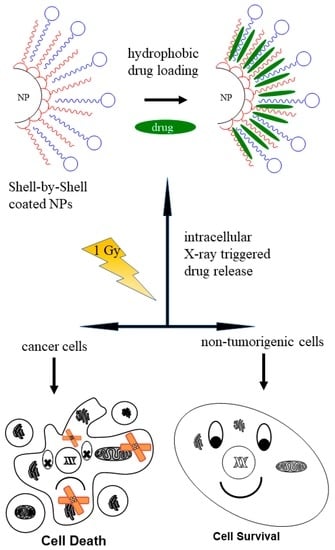
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Surface Functionalization of Nanoparticles
2.3.1. Experimental Procedure for the Preparation of Shell-by-Shell (SbS) Coated Nanoparticle Hybrids
2.3.2. Incorporation of Hydrophobic Molecules
2.4. Cell Culture
2.5. Cell Preparation for Microscope Images
2.5.1. TEM Images
2.5.2. Fluorescent Microscope
2.6. MTT Assay
2.7. Radical Detection Assays
2.7.1. Superoxide
2.7.2. ROS
2.8. Mitochondrial Depolarization
2.9. TUNEL Assay
2.10. Clonogenic Cell Survival Assay
3. Results and Discussion
3.1. Characterization of the Shell-by-Shell Coated Nanocarriers
3.2. Cellular Uptake of the Nanocarriers
3.3. Biocompatibility before and after X-Ray Radiation
3.4. Changes of ROS and Superoxide Concentration
3.5. Mitochondrial Membrane Potential and DNA Fragmentation
3.6. Survival Curves of the Cells with and without Nanocarriers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Venkatasubbu, G.D.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Folate targeted PEGylated titanium dioxide nanoparticles as a nanocarrier for targeted paclitaxel drug delivery. Adv. Powder Technol. 2013, 24, 947–954. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Lu, X.; Tian, H.; Jiang, H.; Lv, G.; Guo, D.; Wu, C.; Chen, B. The incorporation of daunorubicin in cancer cells through the use of titanium dioxide whiskers. Biomaterials 2009, 30, 4708–4715. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 2, 133–149. [Google Scholar]
- Nakamuro, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottesman, M.M.; Pastan, I.; Ambudkar, S.V. P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 1996, 6, 610–617. [Google Scholar] [CrossRef]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 2000, 85, 217–229. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Arun Seshan, C.; Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloid Surf. B 2016, 148, 600–606. [Google Scholar] [CrossRef]
- Fabian, E.; Landsiedel, R.; Ma-Hock, L.; Wiench, K.; Wohlleben, W.; van Ravenzwaay, B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rat. Arch. Toxicol. 2008, 82, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Fartkhooni, F.M.; Norri, A.; Mohammadi, A. Effects of Titanium Dioxide Nanoparticles Toxicity on the Kidney of Male rats. Int. J. Life Sci. 2016, 10, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Shi, H.; Ruth, M.; Yu, H.; Lazar, L.; Zou, B.; Yang, C.; Wu, A.; Zhao, J. Acute Toxicity of Intravenously Administered Titanium Dioxide Nanoparticles in Mice. PLoS ONE 2013, 8, e70618. [Google Scholar] [CrossRef] [Green Version]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeter. Biodegr. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Mazurkova, N.A.; Spitsyna, E.; Shikina, N.V.; Ismagilov, Z.R.; Zagrebel’nyi, S.N.; Ryabchikova, E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russ. 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Çeşmeli, S.; Avci, C.B. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wan, Y.; Wang, Y.; Zhang, H.; Jiao, Z. Anticancer efficacy enhancement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int. J. Nanomed. 2011, 6, 2321–2326. [Google Scholar]
- Zhang, H.; Wang, C.; Chen, B.; Wang, X. Daunorubicin-TiO2 nanocomposites as a “smart” pH-responsive drug delivery system. Int. J. Nanomed. 2012, 7, 235–242. [Google Scholar] [CrossRef]
- Zeininger, L.; Petzi, S.; Schönamsgruber, J.; Portilla, L.; Halik, M.; Hirsch, A. Very Facile Polarity Umpolung and Noncovalent Functionalization of Inorganic Nanoparticles: A Tool Kit for Supramolecular Materials Chemistry. Chem. Eur. J. 2015, 21, 14030–14035. [Google Scholar] [CrossRef]
- Stiegler, L.M.S.; Luchs, T.; Hirsch, A. Shell-by-Shell Functionalization of Inorganic Nanoparticles. Chem. Eur. J. 2020, 26, 8483–8498. [Google Scholar] [CrossRef]
- Stiegler, L.M.S.; Hirsch, A. Electronic Communication in Confined Space Coronas of Shell-by-Shell Structured Al2O3 Nanoparticle Hybrids Containing Two Layers of Functional Organic Ligands. Chem. Eur. J. 2019, 25, 11864–11875. [Google Scholar] [CrossRef] [PubMed]
- Zeininger, L.; Stiegler, L.M.S.; Portilla, L.; Halik, M.; Hirsch, A. Manufacturing Nanoparticles with Orthogonally Adjustable Dispersibility in Hydrocarbons, Fluorocarbons and Water. ChemistryOpen 2018, 7, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Luchs, T.; Sarcletti, M.; Zeininger, L.; Portilla, L.; Fischer, C.; Harder, S.; Halik, M.; Hirsch, A. Highly Efficient Encapsulation and Phase Separation of Apolar Molecules by Magnetic Shell-by-Shell-Coated Nanocarriers in Water. Chem. Eur. J. 2018, 24, 13589–13595. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Dasdashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathways of Apoptosis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-H.; An, J.Y.; Kwon, Y.T.; Rhe, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klenkar, J.; Molnar, M. Natural and synthetic coumarins as potential anticancer drugs. J. Chem. Pharm. Res. 2015, 7, 1223–1238. [Google Scholar]
- Chuang, J.-Y.; Huang, Y.-F.; Lu, H.-F.; Ho, H.-C.; Yang, J.-S.; Li, T.-M.; Chang, N.-W.; Chung, J.-G. Coumarin Induces Cell Cycle Arrest and Apoptosis in Human Cervical Cancer HeLa Cells through a Mitochondria- and Caspase-3 Dependent Mechanism and NF-κB Down-regulation. In Vivo 2007, 21, 1003–1010. [Google Scholar]
- Robaszkiewicz, A.; Balcerczyk, A.; Bartosz, G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol. Int. 2007, 31, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi-Pour, Z.; Ramezani, F.; Keshavarzi, F.; Samadi, N. The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncol. Lett. 2017, 13, 1965–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Mirjolet, C.; Papa, A.L.; Créhange, G.; Raguin, O.; Seignez, C.; Paul, C.; Truc, G.; Maingon, P.N. Millot The radiosensitization effect of titanate nanotubes as a new toll in radiation therapy for glioblastoma: A proof-of-concept. Radiother. Oncol. 2013, 108, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Youkhana, E.Q.; Feltis, B.; Blencowe, A.; Geso, M. Titanium Dioxide Nanoparticles as Radiosensitizer: An In Vitro and Phantom-Based Study. Int. J. Med. Sci. 2017, 14, 602–614. [Google Scholar] [CrossRef] [Green Version]
- Rezaei-Tavirani, M.; Dolat, E.; Hasanzadeh, H.; Seyyedi, S.-S.; Semnani, V.; Sobhi, S. TiO2 Nanoparticle as a Sensitizer Drug in Radiotherapy: In Vitro Study. Iran. J. Cancer Prev. 2013, 6, 37–44. [Google Scholar]
- Babaei, M.; Ganjalikhani, M. The potential effectiveness of nanoparticles as radio sensitizers for radiotherapy. BioImpacts: BI 2014, 4, 15–20. [Google Scholar]
- Langhals, H.; Gold, J. An Unusual β-Oxidation of N-Functionalized Alkyl Chains by 1H-Imidazole. Helv. Chim. Acta 2005, 88, 2832–2836. [Google Scholar] [CrossRef]
- Zeininger, L.; Portilla, L.; Halik, M.; Hirsch, A. Quantitative Determination and Comparison of the Surface Binding of Phosphonic Acid, Carboxylic Acid, and Catechol Ligands on TiO2 Nanoparticles. Chem. Eur. J. 2016, 22, 13506–13512. [Google Scholar] [CrossRef]
- Klein, S.; Kizaloğlu, M.; Portilla, L.; Park, H.; Rejek, T.; Hümmer, J.; Meyer, K.; Hock, R.; Distel, L.V.R.; Halik, M.; et al. Enhanced In Vitro Biocompatibility and Water Dispersibility of Magnetite and Cobalt Ferrite Nanoparticles Employed as ROS Formation Enhancer in Radiation Cancer Therapy. Small 2018, 14, 1704111. [Google Scholar] [CrossRef]
- Klein, S.; Smuda, M.; Harreiß, C.; Menter, C.; Distel, L.V.R.; Kryschi, C. Bifunctional Au-Fe3O4 Nanoheterodimers Acting as X-ray Protector in Healthy Cells and as X-ray Enhancer in Tumor Cells. ACS Appl. Mater. Interfaces 2019, 11, 39613–39623. [Google Scholar] [CrossRef] [PubMed]
- Franke, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Chompoosor, A.; You, C.C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic Pathways and Endosomal Trafficking: A Primer. Wien. Med. Wochenschr. 2016, 166, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Sommer, A.; Distel, L.V.R.; Neuhuber, W.; Kryschi, C. Superparamagnetic iron oxide nanoparticles as radiosensitizers via enhanced reactive oxygen species formation. Biochem. Biophys. Res. Commun. 2012, 425, 393–397. [Google Scholar] [CrossRef]
- Klein, S.; Sommer, A.; Distel, L.V.R.; Hazemann, J.-L.; Kröner, W.; Neuhuber, W.; Müller, P.; Proux, O.; Kryschi, C. Superparamagnetic Iron Oxide Nanoparticles as Novel X-ray Enhancer for Low-Dose Radiation Therapy. J. Phys. Chem. B 2014, 118, 6159–6166. [Google Scholar] [CrossRef]
- Jamová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3’,4’-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Yu, W.; Liu, Z.-L. Antioxidative and prooxidative effects of coumarin derivatives on free radical initiated and photosensitized peroxidation of human low-density lipoprotein. Chem. Phys. Lipids 1999, 103, 125–135. [Google Scholar] [CrossRef]
- Yuting, C.; Rongliang, Z.; Zhongjian, J.; Yong, J. Flavonoids as superoxide scavengers and antioxidants. Free Radical. Bio. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Ueno, I.; Kohno, M.; Haraikawa, K.; Hirono, I. Interactions between Quercetin and Superoxide Radicals. Reduction of the Quercetin Mutagenicity. J. Parmacobiodyn. 1984, 11, 798–803. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef]
- Kam, W.W.-Y.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radical. Bio. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef]
- Hashimoto, M.; Imazato, S. Cytotoxic and genotoxic characterization of aluminium and silicon oxide nanoparticles in macrophages. Dent. Mater. 2015, 31, 556–564. [Google Scholar] [CrossRef]
- Balasubramanyam, A.; Sailaja, N.; Mahboob, M.; Rahman, M.F.; Hussain, S.M.; Grover, P. In vivo genotoxicity assassment of aluminium oxide nanomaterials in rat peripheral blood cells using comet assay and micronucleus test. Mutagenesis 2009, 24, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Vimala, D.S.; Murugesan, R.; Francesco, M.; Antara, B.; Xiao, F.S.; Surajit, P. Comparative study on anti-proliferative potentials of zinc oxide and aluminium oxide nanoparticles in colon cancer cells. Acta Biomed. 2019, 90, 241–247. [Google Scholar]
- Alshatwi, A.A.; Subbarayan, P.V.; Ramesh, E.; Al-Hazzani, A.A.; Alsaif, M.A.; Alwarthan, A.A. Aluminium oxide nanoparticles induce mitoxhondrial-mediated oxidative stress and alter the expression of antioxidant enzymes in human mesenchymal stem cells. Food Addit. Contam. Part A 2013, 30. [Google Scholar] [CrossRef]
- Manning, S.J.; Bogen, W.; Kelly, L.A. Synthesis, Characterization, and Photophysical Study of Fluorescent N-substituted Benzo[ghi]perylene “Swallow Tail” Monoimides. J. Org. Chem. 2011, 76, 6007–6013. [Google Scholar] [CrossRef] [PubMed]









| Nanocarrier | Size in Water (nm) | Size in Medium (nm) | Zeta Potential (mV) | LC (%) |
|---|---|---|---|---|
| [TiO2-PAC16]@guest G1@shell1 | 106 | 197 | −23.6 ± 2.2 | 7.1 |
| [TiO2-PAC16]@guest G2@shell1 | 115 | 209 | −38.5 ± 1.9 | |
| [TiO2-PAC16]@guest G3@shell1 | 100 | 195 | −22.3 ± 1.0 | 3.1 |
| [Al2O3-PAC16]@guest G1@shell1 | 130 | 186 | −35.0 ± 2.8 | 4.6 |
| [Al2O3-PAC16]@guest G3@shell1 | 143 | 194 | −35.0 ± 2.8 | 5.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, S.; Luchs, T.; Leng, A.; Distel, L.V.R.; Neuhuber, W.; Hirsch, A. Encapsulation of Hydrophobic Drugs in Shell-by-Shell Coated Nanoparticles for Radio—and Chemotherapy—An In Vitro Study. Bioengineering 2020, 7, 126. https://doi.org/10.3390/bioengineering7040126
Klein S, Luchs T, Leng A, Distel LVR, Neuhuber W, Hirsch A. Encapsulation of Hydrophobic Drugs in Shell-by-Shell Coated Nanoparticles for Radio—and Chemotherapy—An In Vitro Study. Bioengineering. 2020; 7(4):126. https://doi.org/10.3390/bioengineering7040126
Chicago/Turabian StyleKlein, Stefanie, Tobias Luchs, Andreas Leng, Luitpold V. R. Distel, Winfried Neuhuber, and Andreas Hirsch. 2020. "Encapsulation of Hydrophobic Drugs in Shell-by-Shell Coated Nanoparticles for Radio—and Chemotherapy—An In Vitro Study" Bioengineering 7, no. 4: 126. https://doi.org/10.3390/bioengineering7040126
APA StyleKlein, S., Luchs, T., Leng, A., Distel, L. V. R., Neuhuber, W., & Hirsch, A. (2020). Encapsulation of Hydrophobic Drugs in Shell-by-Shell Coated Nanoparticles for Radio—and Chemotherapy—An In Vitro Study. Bioengineering, 7(4), 126. https://doi.org/10.3390/bioengineering7040126