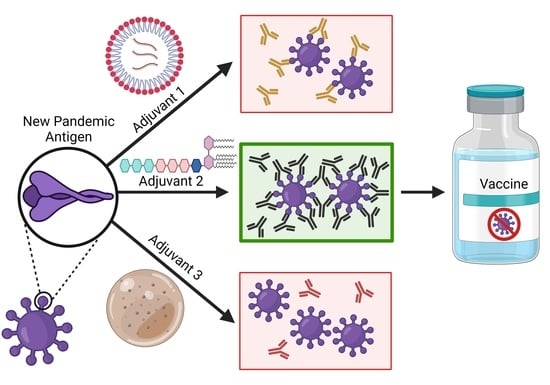
Novel Vaccine Adjuvants as Key Tools for Improving Pandemic Preparedness
Abstract
:1. Introduction
2. Adjuvants in Clinically Approved Vaccines
3. Shortcomings of Current Adjuvants
4. Adjuvants with New Targets
5. Emerging Adjuvant Platforms
6. Discussion: Perspectives for Adjuvant Translation to Pandemic Preparedness
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 14 September 2021).
- Augustin, M.; Schommers, P.; Suárez, I.; Koehler, P.; Gruell, H.; Klein, F.; Maurer, C.; Langerbeins, P.; Priesner, V.; Schmidt-Hellerau, K.; et al. Rapid Response Infrastructure for Pandemic Preparedness in a Tertiary Care Hospital: Lessons Learned from the COVID-19 Outbreak in Cologne, Germany, February to March 2020. Eurosurveillance 2020, 25, 2000531. [Google Scholar] [CrossRef]
- Weintraub, R.; Plotkin, S.; Liu, M.; Kim, J.; Garcon, N.; Bell, D.; Storisteanu, D.; Norman, T.; Aronoff-Spencer, E. COVID-19 Vaccine Delivery: An Opportunity to Set up Systems for the Future 2021. Gates Open Res. 2021, 4, 182. [Google Scholar] [CrossRef]
- Webb, S.R.; Twyman, R.M.; Moloney, M. Agtech Infrastructure for Pandemic Preparedness. Nat. Biotechnol. 2020, 38, 1025–1027. [Google Scholar] [CrossRef]
- Possas, C.; de Souza Antunes, A.M.; de Oliveira, A.M.; de Souza Mendes Santos, C.D.U.; Ramos, M.P.; de Oliveira Rodrigues Schumacher, S.; Homma, A. Vaccine Innovation for Pandemic Preparedness: Patent Landscape, Global Sustainability, and Circular Bioeconomy in Post-COVID-19 Era. Circ. Econ. Sustain. 2021, 1–23. [Google Scholar] [CrossRef]
- Marston, H.D.; Paules, C.I.; Fauci, A.S. The Critical Role of Biomedical Research in Pandemic Preparedness. JAMA 2017, 318, 1757–1758. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, S.P.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Ho, R.J.Y. Warp-Speed COVID-19 Vaccine Development: Beneficiaries of Maturation in Biopharmaceutical Technologies and Public-Private Partnerships. J. Pharm. Sci. 2021, 110, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Garcon, N.; Friede, M. Evolution of Adjuvants Across the Centuries. In Plotkin’s Vaccines; Elesevier: Philadelphia, PA, USA, 2018; pp. 61–74. ISBN 978-0-323-35761-6. [Google Scholar]
- Dumpa, N.; Goel, K.; Guo, Y.; McFall, H.; Pillai, A.R.; Shukla, A.; Repka, M.A.; Murthy, S.N. Stability of Vaccines. AAPS PharmSciTech 2019, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Lin, X.; Li, Z.; Ma, G.; Su, Z.; Zhang, S. Biocompatible Cationic Solid Lipid Nanoparticles as Adjuvants Effectively Improve Humoral and T Cell Immune Response of Foot and Mouth Disease Vaccines. Vaccine 2020, 38, 2478–2486. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A. Decoding the Patterns of Self and Nonself by the Innate Immune System. Science 2002, 296, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R.; Janeway, C.A. Innate Immunity: The Virtues of a Nonclonal System of Recognition. Cell 1997, 91, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, S.A. Vaccines: Past, Present and Future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Corry, D.B.; Strych, U.; Bottazzi, M.E. COVID-19 Vaccines: Neutralizing Antibodies and the Alum Advantage. Nat. Rev. Immunol. 2020, 20, 399–400. [Google Scholar] [CrossRef]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA Released from Dying Host Cells Mediates Aluminum Adjuvant Activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, N.W.; Malinczak, C.-A. Harnessing Cellular Immunity for Vaccination against Respiratory Viruses. Vaccines 2020, 8, 783. [Google Scholar] [CrossRef]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel Cellular and Molecular Mechanisms of Induction of Immune Responses by Aluminum Adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef]
- Clapp, T.; Siebert, P.; Chen, D.; Jones Braun, L. Vaccines with Aluminum-Containing Adjuvants: Optimizing Vaccine Efficacy and Thermal Stability. J. Pharm. Sci. 2011, 100, 388–401. [Google Scholar] [CrossRef] [Green Version]
- O’Hagan, D.T.; Lodaya, R.N.; Lofano, G. The Continued Advance of Vaccine Adjuvants—‘We Can Work It Out’. Semin. Immunol. 2020, 50, 101426. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.; Hartmann, K.; Künzi, V.; Kürsteiner, O.; Mischler, R.; Lazar, H.; Glück, R. Eleven Years of Inflexal® V—A Virosomal Adjuvanted Influenza Vaccine. Vaccine 2009, 27, 4381–4387. [Google Scholar] [CrossRef]
- Petkar, K.C.; Patil, S.M.; Chavhan, S.S.; Kaneko, K.; Sawant, K.K.; Kunda, N.K.; Saleem, I.Y. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics 2021, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of Nanotechnology behind the Success of MRNA Vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef] [PubMed]
- Hartman, Z.C.; Appledorn, D.M.; Amalfitano, A. Adenovirus Vector Induced Innate Immune Responses: Impact upon Efficacy and Toxicity in Gene Therapy and Vaccine Applications. Virus Res. 2008, 132, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, Y.; Feng, L.; Pan, W.; Zhang, M.; Hong, Z.; Ma, X.; Chen, X.; Chen, L. Epidemiology of Adenovirus Type 5 Neutralizing Antibodies in Healthy People and AIDS Patients in Guangzhou, Southern China. Vaccine 2011, 29, 3837–3841. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The Mechanism of Action of MF59—An Innately Attractive Adjuvant Formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Seubert, A.; Calabro, S.; Santini, L.; Galli, B.; Genovese, A.; Valentini, S.; Aprea, S.; Colaprico, A.; D’Oro, U.; Giuliani, M.M.; et al. Adjuvanticity of the Oil-in-Water Emulsion MF59 Is Independent of Nlrp3 Inflammasome but Requires the Adaptor Protein MyD88. Proc. Natl. Acad. Sci. USA 2011, 108, 11169–11174. [Google Scholar] [CrossRef] [Green Version]
- Banzhoff, A.; Gasparini, R.; Laghi-Pasini, F.; Staniscia, T.; Durando, P.; Montomoli, E.; Capecchi, P.; Giovanni, P.D.; Sticchi, L.; Gentile, C.; et al. MF59®-Adjuvanted H5N1 Vaccine Induces Immunologic Memory and Heterotypic Antibody Responses in Non-Elderly and Elderly Adults. PLoS ONE 2009, 4, e4384. [Google Scholar] [CrossRef]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 Containing α-Tocopherol Modulates Innate Immune Response and Leads to Improved Adaptive Immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef]
- Kim, E.H.; Woodruff, M.C.; Grigoryan, L.; Maier, B.; Lee, S.H.; Mandal, P.; Cortese, M.; Natrajan, M.S.; Ravindran, R.; Ma, H.; et al. Squalene Emulsion-Based Vaccine Adjuvants Stimulate CD8 T Cell, but Not Antibody Responses, through a RIPK3-Dependent Pathway. eLife 2020, 9, e52687. [Google Scholar] [CrossRef] [PubMed]
- Giarola-Silva, S.; Coelho-dos-Reis, J.G.A.; Mourão, M.M.; Campi-Azevedo, A.C.; Nakagaki Silva, E.E.; Luiza-Silva, M.; Martins, M.A.; Silveira-Cassette, A.C.D.O.; Batista, M.A.; Peruhype-Magalhães, V.; et al. Distinct Patterns of Cellular Immune Response Elicited by Influenza Non-Adjuvanted and AS03-Adjuvanted Monovalent H1N1(Pdm09) Vaccine. Antivir. Res. 2017, 144, 70–82. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like Receptor Control of the Adaptive Immune Responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.F.; Coviello, S.; Monsalvo, A.C.; Melendi, G.A.; Hernandez, J.Z.; Batalle, J.P.; Diaz, L.; Trento, A.; Chang, H.-Y.; Mitzner, W.; et al. Lack of Antibody Affinity Maturation Due to Poor Toll-like Receptor Stimulation Leads to Enhanced Respiratory Syncytial Virus Disease. Nat. Med. 2009, 15, 34–41. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Huffel, C.V.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casella, C.R.; Mitchell, T.C. Putting Endotoxin to Work for Us: Monophosphoryl Lipid A as a Safe and Effective Vaccine Adjuvant. Cell. Mol. Life Sci. 2008, 65, 3231. [Google Scholar] [CrossRef] [Green Version]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant System AS01: Helping to Overcome the Challenges of Modern Vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a Vaccine Adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Siegrist, C.-A. Vaccine Immunology. In Plotkin’s Vaccines, 7th ed.; Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 16–34.e7. ISBN 978-0-323-35761-6. [Google Scholar]
- Lee, P.; Kim, C.-U.; Seo, S.H.; Kim, D.-J. Current Status of COVID-19 Vaccine Development: Focusing on Antigen Design and Clinical Trials on Later Stages. Immune Netw. 2021, 21, e4. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; Paula, R.C.D.; Paula, O.F.P.D.; Villela, E.F.D.M.; Torres, M.S.S.; Oliveira, S.B.D.; Schulz, W.; et al. Effectiveness of the CoronaVac Vaccine in Older Adults during a Gamma Variant Associated Epidemic of COVID-19 in Brazil: Test Negative Case-Control Study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; Dean, N.E.; Madewell, Z.J.; Yang, Y.; Halloran, M.E.; Longini, I. Efficacy Estimates for Various COVID-19 Vaccines: What We Know from the Literature and Reports. medRxiv 2021. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinto, H.; Otieno, W.; Gesase, S.; Sorgho, H.; Otieno, L.; Liheluka, E.; Valéa, I.; Sing’oei, V.; Malabeja, A.; Valia, D.; et al. Long-Term Incidence of Severe Malaria Following RTS,S/AS01 Vaccination in Children and Infants in Africa: An Open-Label 3-Year Extension Study of a Phase 3 Randomised Controlled Trial. Lancet Infect. Dis. 2019, 19, 821–832. [Google Scholar] [CrossRef]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/covid-vaccinations (accessed on 16 September 2021).
- Lloyd, J.; Cheyne, J. The Origins of the Vaccine Cold Chain and a Glimpse of the Future. Vaccine 2017, 35, 2115–2120. [Google Scholar] [CrossRef]
- Kumru, O.S.; Joshi, S.B.; Smith, D.E.; Middaugh, C.R.; Prusik, T.; Volkin, D.B. Vaccine Instability in the Cold Chain: Mechanisms, Analysis and Formulation Strategies. Biologicals 2014, 42, 237–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heineman, T.C.; Cunningham, A.; Levin, M. Understanding the Immunology of Shingrix, a Recombinant Glycoprotein E Adjuvanted Herpes Zoster Vaccine. Curr. Opin. Immunol. 2019, 59, 42–48. [Google Scholar] [CrossRef]
- Levin, M.J.; Kroehl, M.E.; Johnson, M.J.; Hammes, A.; Reinhold, D.; Lang, N.; Weinberg, A. Th1 Memory Differentiates Recombinant from Live Herpes Zoster Vaccines. J. Clin. Investig. 2018, 128, 4429–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogoi, H.; Mansouri, S.; Jin, L. The Age of Cyclic Dinucleotide Vaccine Adjuvants. Vaccines 2020, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Xia, N.; Zhao, Q. Carbohydrate-Containing Nanoparticles as Vaccine Adjuvants. Expert Rev. Vaccines 2021, 20, 797–810. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Shibu, M.A.; Toth, I.; Skwarczynskia, M. Cell-Penetrating Peptides: Efficient Vectors for Vaccine Delivery. Curr. Drug Deliv. 2019, 16, 430–443. [Google Scholar] [CrossRef]
- Nagpal, G.; Chaudhary, K.; Agrawal, P.; Raghava, G.P.S. Computer-Aided Prediction of Antigen Presenting Cell Modulators for Designing Peptide-Based Vaccine Adjuvants. J. Transl. Med. 2018, 16, 181. [Google Scholar] [CrossRef] [Green Version]
- Baindara, P.; Chakraborty, R.; Holliday, Z.M.; Mandal, S.M.; Schrum, A.G. Oral Probiotics in Coronavirus Disease 2019: Connecting the Gut–Lung Axis to Viral Pathogenesis, Inflammation, Secondary Infection and Clinical Trials. New Microbes New Infect. 2021, 40, 100837. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Jeon, Y.J.; Namgung, B.; Hong, M.; Yoon, S. A Conserved TLR5 Binding and Activation Hot Spot on Flagellin. Sci. Rep. 2017, 7, 40878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Pan, Z.; Kang, X.; Yang, Y.; Kang, H.; Zhang, N.; Rosati, J.M.; Jiao, X. Amino Acids 89–96 of Salmonella Typhimurium Flagellin Represent the Major Domain Responsible for TLR5-Independent Adjuvanticity in the Humoral Immune Response. Cell. Mol. Immunol. 2015, 12, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacchieri, S.G.; Torquato, R.; Brentani, R.R. Structural Study of Binding of Flagellin by Toll-Like Receptor 5. J. Bacteriol. 2003, 185, 4243–4247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaves-Pyles, T.D.; Wong, H.R.; Odoms, K.; Pyles, R.B. Salmonella Flagellin-Dependent Proinflammatory Responses Are Localized to the Conserved Amino and Carboxyl Regions of the Protein. J. Immunol. 2001, 167, 7009–7016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Means, T.K.; Hayashi, F.; Smith, K.D.; Aderem, A.; Luster, A.D. The Toll-Like Receptor 5 Stimulus Bacterial Flagellin Induces Maturation and Chemokine Production in Human Dendritic Cells. J. Immunol. 2003, 170, 5165–5175. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Carvalho, F.A.; Aitken, J.D.; Fifadara, N.H.; Gewirtz, A.T. TLR5 or NLRC4 Is Necessary and Sufficient for Promotion of Humoral Immunity by Flagellin. Eur. J. Immunol. 2010, 40, 3528–3534. [Google Scholar] [CrossRef] [Green Version]
- Cuadros, C.; Lopez-Hernandez, F.J.; Dominguez, A.L.; McClelland, M.; Lustgarten, J. Flagellin Fusion Proteins as Adjuvants or Vaccines Induce Specific Immune Responses. Infect. Immun. 2004, 72, 2810–2816. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.-J.; Lee, Y.; Lee, Y.-T.; Jung, Y.-J.; Ngo, V.L.; Kim, M.-C.; Kim, K.-H.; Wang, B.-Z.; Gewirtz, A.T.; Kang, S.-M. Flagellin-Expressing Virus-like Particles Exhibit Adjuvant Effects on Promoting IgG Isotype-Switched Long-Lasting Antibody Induction and Protection of Influenza Vaccines in CD4-Deficient Mice. Vaccine 2019, 37, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Zhu, X.; Cao, Y.; Chen, X. Improving Immunogenicity and Safety of Flagellin as Vaccine Carrier by High-Density Display on Virus-like Particle Surface. Biomaterials 2020, 249, 120030. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Lottenbach, K.; Graham, I.; Anderson, E.; Bajwa, K.; May, R.C.; Mizel, S.B.; Graff, A.; Belshe, R.B. A Phase I Safety and Immunogenicity Dose Escalation Trial of Plague Vaccine, Flagellin/F1/V, in Healthy Adult Volunteers (DMID 08-0066). Vaccine 2017, 35, 6759–6765. [Google Scholar] [CrossRef]
- Khim, K.; Bang, Y.J.; Puth, S.; Choi, Y.; Lee, Y.S.; Jeong, K.; Lee, S.E.; Rhee, J.H. Deimmunization of Flagellin for Repeated Administration as a Vaccine Adjuvant. Npj Vaccines 2021, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Varshney, D.; Qiu, S.Y.; Graf, T.P.; McHugh, K.J. Employing Drug Delivery Strategies to Overcome Challenges Using TLR7/8 Agonists for Cancer Immunotherapy. AAPS J. 2021, 23, 90. [Google Scholar] [CrossRef]
- Lynn, G.M.; Sedlik, C.; Baharom, F.; Zhu, Y.; Ramirez-Valdez, R.A.; Coble, V.L.; Tobin, K.; Nichols, S.R.; Itzkowitz, Y.; Zaidi, N.; et al. Peptide–TLR-7/8a Conjugate Vaccines Chemically Programmed for Nanoparticle Self-Assembly Enhance CD8 T-Cell Immunity to Tumor Antigens. Nat. Biotechnol. 2020, 38, 320–332. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Lu, S.; Igweze, J.; Xu, W.; Kuang, D.; Zealey, C.; Liu, D.; Gregor, A.; Bozorgzad, A.; et al. Self-Assembling Peptide for Co-Delivery of HIV-1 CD8+ T Cells Epitope and Toll-like Receptor 7/8 Agonists R848 to Induce Maturation of Monocyte Derived Dendritic Cell and Augment Polyfunctional Cytotoxic T Lymphocyte (CTL) Response. J. Control. Release 2016, 236, 22–30. [Google Scholar] [CrossRef]
- Mehravaran, A.; Mirahmadi, H.; Akhtari, J. Liposomes Containing the Imiquimod Adjuvant as a Vaccine in the Cutaneous Leishmaniasis Model. Nanomed. J. 2020, 7, 29–39. [Google Scholar]
- El Sahly, H.M.; Atmar, R.L.; Sendra, E.; Wegel, A.; Keitel, W.A. Topical Imiquimod Does Not Provide an Adjuvant Effect When Administered with Inactivated Influenza A/H5N1 Vaccine in Healthy Young Adults. J. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Mombelli, M.; Hoschler, K.; Cavassini, M.; Pascual, M.; Manuel, O. Seasonal Trivalent Inactivated Influenza Vaccine with Topical Imiquimod in Immunocompromised Patients: A Randomized Controlled Trial. J. Infect. 2021, 83, 354–360. [Google Scholar] [CrossRef]
- Dowling, D.J. Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. ImmunoHorizons 2018, 2, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Manes, N.P.; Nita-Lazar, A. Molecular Mechanisms of the Toll-Like Receptor, STING, MAVS, Inflammasome, and Interferon Pathways. mSystems 2021, 6, e00336-21. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Akira, S. Identification and Functions of Pattern-Recognition Receptors. J. Allergy Clin. Immunol. 2010, 125, 985–992. [Google Scholar] [CrossRef]
- Rai, R.C. Host Inflammatory Responses to Intracellular Invaders: Review Study. Life Sci. 2020, 240, 117084. [Google Scholar] [CrossRef]
- Gray, P.M.; Forrest, G.; Wisniewski, T.; Porter, G.; Freed, D.C.; DeMartino, J.A.; Zaller, D.M.; Guo, Z.; Leone, J.; Fu, T.-M.; et al. Evidence for Cyclic Diguanylate as a Vaccine Adjuvant with Novel Immunostimulatory Activities. Cell. Immunol. 2012, 278, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-Silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled Release of a Cyclic Dinucleotide for Enhanced Cancer Immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING Agonists Are Potent Lymph Node–Targeted Vaccine Adjuvants. J. Clin. Investig. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dis, E.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium Tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef]
- Aroh, C.; Wang, Z.; Dobbs, N.; Luo, M.; Chen, Z.; Gao, J.; Yan, N. Innate Immune Activation by CGMP-AMP Nanoparticles Leads to Potent and Long-Acting Antiretroviral Response against HIV-1. J. Immunol. 2017, 199, 3840–3848. [Google Scholar] [CrossRef] [Green Version]
- Koshy, S.T.; Cheung, A.S.; Gu, L.; Graveline, A.R.; Mooney, D.J. Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Adv. Biosyst. 2017, 1, 1600013. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Dharmaraj, N.; Lopez-Silva, T.L.; Venzor, J.R.; Pogostin, B.H.; Sikora, A.G.; Hartgerink, J.D.; Young, S. Biomaterial-Facilitated Immunotherapy for Established Oral Cancers. ACS Biomater. Sci. Eng. 2021, 7, 415–421. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through C-Type Lectin Receptors: Shaping Immune Responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Miller, J.L.; deWet, B.J.M.; Martinez-Pomares, L.; Radcliffe, C.M.; Dwek, R.A.; Rudd, P.M.; Gordon, S. The Mannose Receptor Mediates Dengue Virus Infection of Macrophages. PLoS Pathog. 2008, 4, e17. [Google Scholar] [CrossRef]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Wandall, H.H.; Belleudi, F.; Bellati, F.; Pierelli, L.; et al. Targeting of Macrophage Galactose-Type C-Type Lectin (MGL) Induces DC Signaling and Activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Tada, H.; Nemoto, E.; Shimauchi, H.; Watanabe, T.; Mikami, T.; Matsumoto, T.; Ohno, N.; Tamura, H.; Shibata, K.; Akashi, S.; et al. Saccharomyces Cerevisiae- and Candida Albicans-Derived Mannan Induced Production of Tumor Necrosis Factor Alpha by Human Monocytes in a CD14- and Toll-Like Receptor 4-Dependent Manner. Microbiol. Immunol. 2002, 46, 503–512. [Google Scholar] [CrossRef]
- Bermejo-Jambrina, M.; Eder, J.; Helgers, L.C.; Hertoghs, N.; Nijmeijer, B.M.; Stunnenberg, M.; Geijtenbeek, T.B.H. C-Type Lectin Receptors in Antiviral Immunity and Viral Escape. Front. Immunol. 2018, 9, 590. [Google Scholar] [CrossRef]
- Lampe, A.T.; Farris, E.J.; Brown, D.M.; Pannier, A.K. High- and Low-molecular-weight Chitosan Act as Adjuvants during Single-dose Influenza A Virus Protein Vaccination through Distinct Mechanisms. Biotechnol. Bioeng. 2021, 118, 1224–1243. [Google Scholar] [CrossRef]
- Bashiri, S.; Koirala, P.; Toth, I.; Skwarczynski, M. Carbohydrate Immune Adjuvants in Subunit Vaccines. Pharmaceutics 2020, 12, 965. [Google Scholar] [CrossRef]
- Liao, J.; Pan, B.; Liao, G.; Zhao, Q.; Gao, Y.; Chai, X.; Zhuo, X.; Wu, Q.; Jiao, B.; Pan, W.; et al. Synthesis and Immunological Studies of β-1,2-Mannan-Peptide Conjugates as Antifungal Vaccines. Eur. J. Med. Chem. 2019, 173, 250–260. [Google Scholar] [CrossRef]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and Synthetic Carbohydrate-Based Vaccine Adjuvants and Their Mechanisms of Action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Qi, J.; He, Y.; Shen, L.; Yu, W.; Hu, T. Conjugation of Hemoglobin and Mannan Markedly Improves the Immunogenicity of Domain III of the Zika Virus E Protein: Structural and Immunological Study. Bioconjug. Chem. 2021, 32, 328–338. [Google Scholar] [CrossRef]
- Chanput, W.; Reitsma, M.; Kleinjans, L.; Mes, J.J.; Savelkoul, H.F.J.; Wichers, H.J. β-Glucans Are Involved in Immune-Modulation of THP-1 Macrophages. Mol. Nutr. Food Res. 2012, 56, 822–833. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Liang, X.; Li, L.; Gong, S.; Li, X.; Zhang, M.; Zhu, S.; Xiao, H.; Wu, Q.; Gong, C. A Spontaneously Formed and Self-Adjuvanted Hydrogel Vaccine Triggers Strong Immune Responses. Mater. Des. 2021, 197, 109232. [Google Scholar] [CrossRef]
- Vassilaros, S.; Tsibanis, A.; Tsikkinis, A.; Pietersz, G.A.; McKenzie, I.F.; Apostolopoulos, V. Up to 15-Year Clinical Follow-Up of a Pilot Phase III Immunotherapy Study in Stage II Breast Cancer Patients Using Oxidized Mannan–MUC1. Available online: https://www.futuremedicine.com/doi/abs/10.2217/imt.13.126 (accessed on 20 September 2021).
- El-Kamary, S.S.; Pasetti, M.F.; Mendelman, P.M.; Frey, S.E.; Bernstein, D.I.; Treanor, J.J.; Ferreira, J.; Chen, W.H.; Sublett, R.; Richardson, C.; et al. Adjuvanted Intranasal Norwalk Virus-Like Particle Vaccine Elicits Antibodies and Antibody-Secreting Cells That Express Homing Receptors for Mucosal and Peripheral Lymphoid Tissues. J. Infect. Dis. 2010, 202, 1649–1658. [Google Scholar] [CrossRef]
- Garcia-Vello, P.; Speciale, I.; Chiodo, F.; Molinaro, A.; De Castro, C. Carbohydrate-Based Adjuvants. Drug Discov. Today Technol. 2020, 35–36, 57–68. [Google Scholar] [CrossRef]
- Li, W.A.; Lu, B.Y.; Gu, L.; Choi, Y.; Kim, J.; Mooney, D.J. The Effect of Surface Modification of Mesoporous Silica Micro-Rod Scaffold on Immune Cell Activation and Infiltration. Biomaterials 2016, 83, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellacherie, M.O.; Li, A.W.; Lu, B.Y.; Mooney, D.J. Covalent Conjugation of Peptide Antigen to Mesoporous Silica Rods to Enhance Cellular Responses. Bioconjug. Chem. 2018, 29, 733–741. [Google Scholar] [CrossRef]
- Dellacherie, M.O.; Li, A.; Lu, B.Y.; Verbeke, C.S.; Gu, L.; Stafford, A.G.; Doherty, E.J.; Mooney, D.J. Single-Shot Mesoporous Silica Rods Scaffold for Induction of Humoral Responses Against Small Antigens. Adv. Funct. Mater. 2020, 30, 2002448. [Google Scholar] [CrossRef]
- Super, M.; Doherty, E.J.; Cartwright, M.J.; Seiler, B.T.; Langellotto, F.; Dimitrakakis, N.; White, D.A.; Stafford, A.G.; Karkada, M.; Graveline, A.R.; et al. Biomaterial Vaccines Capturing Pathogen-Associated Molecular Patterns Protect against Bacterial Infections and Septic Shock. Nat. Biomed. Eng. 2021, 1–11. [Google Scholar] [CrossRef]
- Abudula, T.; Bhatt, K.; Eggermont, L.J.; O’Hare, N.; Memic, A.; Bencherif, S.A. Supramolecular Self-Assembled Peptide-Based Vaccines: Current State and Future Perspectives. Front. Chem. 2020, 8, 598160. [Google Scholar] [CrossRef]
- Rudra, J.S.; Tian, Y.F.; Jung, J.P.; Collier, J.H. A Self-Assembling Peptide Acting as an Immune Adjuvant. Proc. Natl. Acad. Sci. USA 2010, 107, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Pompano, R.R.; Chen, J.; Verbus, E.A.; Han, H.; Fridman, A.; McNeely, T.; Collier, J.H.; Chong, A.S. Titrating T-Cell Epitopes within Self-Assembled Vaccines Optimizes CD4+ Helper T Cell and Antibody Outputs. Adv. Healthc. Mater. 2014, 3, 1898–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudalla, G.A.; Modica, J.A.; Tian, Y.F.; Rudra, J.S.; Chong, A.S.; Sun, T.; Mrksich, M.; Collier, J.H. A Self-Adjuvanting Supramolecular Vaccine Carrying a Folded Protein Antigen. Adv. Healthc. Mater. 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Fries, C.; Dennis, M.; Eudailey, J.; Moody, A.; Permar, S.; Collier, J.; Fouda, G. Multivalent Antigen Presentation Increases the Antibody Binding Breadth and Neutralizing Potency upon the Immunization with a Self-Assembling HIV Env Vaccine. J. Int. AIDS Soc. 2021, 24, 45–47. [Google Scholar]
- Kelly, S.H.; Opolot, E.E.; Wu, Y.; Cossette, B.; Varadhan, A.K.; Collier, J.H. Tabletized Supramolecular Assemblies for Sublingual Peptide Immunization. Adv. Healthc. Mater. 2021, 10, 2001614. [Google Scholar] [CrossRef]
- Si, Y.; Wen, Y.; Kelly, S.H.; Chong, A.S.; Collier, J.H. Intranasal Delivery of Adjuvant-Free Peptide Nanofibers Elicits Resident CD8+ T Cell Responses. J. Control. Release 2018, 282, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pompano, R.R.; Santiago, F.W.; Maillat, L.; Sciammas, R.; Sun, T.; Han, H.; Topham, D.J.; Chong, A.S.; Collier, J.H. The Use of Self-Adjuvanting Nanofiber Vaccines to Elicit High-Affinity B Cell Responses to Peptide Antigens without Inflammation. Biomaterials 2013, 34, 8776–8785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, S.H.; Wu, Y.; Varadhan, A.K.; Curvino, E.J.; Chong, A.S.; Collier, J.H. Enabling Sublingual Peptide Immunization with Molecular Self-Assemblies. Biomaterials 2020, 241, 119903. [Google Scholar] [CrossRef]
- Sun, T.; Han, H.; Hudalla, G.A.; Wen, Y.; Pompano, R.R.; Collier, J.H. Thermal Stability of Self-Assembled Peptide Vaccine Materials. Acta Biomater. 2016, 30, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, F.C.; Abeyrathna, S.S.; Abeyrathna, N.S.; Wijesundara, Y.H.; Brohlin, O.R.; Carraro, F.; Amenitsch, H.; Falcaro, P.; Luzuriaga, M.A.; Durand-Silva, A.; et al. Stabilization of Supramolecular Membrane Protein–Lipid Bilayer Assemblies through Immobilization in a Crystalline Exoskeleton. Nat. Commun. 2021, 12, 2202. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, C.; Yang, S.; Xiao, W.; Zheng, Q.; Song, X. The Investigation of MRNA Vaccines Formulated in Liposomes Administrated in Multiple Routes against SARS-CoV-2. J. Control. Release 2021, 335, 449–456. [Google Scholar] [CrossRef]
- Alfagih, I.M.; Aldosari, B.; AlQuadeib, B.; Almurshedi, A.; Alfagih, M.M. Nanoparticles as Adjuvants and Nanodelivery Systems for MRNA-Based Vaccines. Pharmaceutics 2021, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for MRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Démoulins, T.; Milona, P.; Englezou, P.C.; Ebensen, T.; Schulze, K.; Suter, R.; Pichon, C.; Midoux, P.; Guzmán, C.A.; Ruggli, N.; et al. Polyethylenimine-Based Polyplex Delivery of Self-Replicating RNA Vaccines. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and Immunogenicity of a MRNA Rabies Vaccine in Healthy Adults: An Open-Label, Non-Randomised, Prospective, First-in-Human Phase 1 Clinical Trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced Intranasal Delivery of MRNA Vaccine by Overcoming the Nasal Epithelial Barrier via Intra- and Paracellular Pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef]
- Gutjahr, A.; Papagno, L.; Nicoli, F.; Lamoureux, A.; Vernejoul, F.; Lioux, T.; Gostick, E.; Price, D.A.; Tiraby, G.; Perouzel, E.; et al. Cutting Edge: A Dual TLR2 and TLR7 Ligand Induces Highly Potent Humoral and Cell-Mediated Immune Responses. J. Immunol. 2017, 198, 4205–4209. [Google Scholar] [CrossRef] [Green Version]
- Pavot, V.; Rochereau, N.; Primard, C.; Genin, C.; Perouzel, E.; Lioux, T.; Paul, S.; Verrier, B. Encapsulation of Nod1 and Nod2 Receptor Ligands into Poly(Lactic Acid) Nanoparticles Potentiates Their Immune Properties. J. Control. Release 2013, 167, 60–67. [Google Scholar] [CrossRef]
- Gutjahr, A.; Phelip, C.; Coolen, A.-L.; Monge, C.; Boisgard, A.-S.; Paul, S.; Verrier, B. Biodegradable Polymeric Nanoparticles-Based Vaccine Adjuvants for Lymph Nodes Targeting. Vaccines 2016, 4, 34. [Google Scholar] [CrossRef]
- Subbaraman, N. The US Is Boosting Funding for Research Monkeys in the Wake of COVID. Nature 2021, 595, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.H.; Nidom, R.V.; Ventura, R.; Indrasari, S.; Normalina, I.; Santoso, K.P.; Derouet, F.; Barnier-Quer, C.; Borchard, G.; Collin, N.; et al. Better Pandemic Influenza Preparedness through Adjuvant Technology Transfer: Challenges and Lessons Learned. Vaccines 2021, 9, 461. [Google Scholar] [CrossRef] [PubMed]


| Adjuvant (Year) | Composition | Licensed Vaccine Targets | Immunological Function |
|---|---|---|---|
| Alum (1926) | Suspension of aluminum hydroxide or aluminum phosphate salts | Anthrax, hepatitis A, hepatitis B, human papillomavirus, diphtheria-pertussis-tetanus (DPT and TdaP), haemophilus influenzae type b, Japanese encephalitis, pneumococcal conjugate vaccines, and COVID-19 | Releases DAMPs at injection site by causing cell death, resulting in the recruitment and activation of dendritic cells and neutrophils |
| Virosomes (1993) | Unilamellar liposomes composed of viral proteins and phospholipids of vaccine target virus | Seasonal flu and hepatitis A | PAMPs on the surface of virosomes stimulate and activate antigen-presenting cells while also facilitating antigen delivery |
| MF59 (1997) | Emulsion of Squalene, Tween (polysorbate) 80, and Span 85 | Seasonal flu and pandemic flu (H1N1) | Known to recruit and activate macrophages and dendritic cells and cause chemokine secretion |
| AS03 (2009) | Emulsion of Squalene, α-tocopherol, and Tween (polysorbate) 80 | H1N1 | Activates human monocytes and macrophages and induces NF-κB activity and chemokine production |
| AS04 (2009) | MPL adsorbed onto alum | Human papillomavirus and hepatitis B | MPL activates TLR4 and NF-κB to stimulate antigen presenting cells and innate immune system while alum causes the release of DAMPs and local inflammation |
| AS01 (2015) | Liposome co-delivery of saponin QS-21 and MPL | Malaria and herpes zoster | Activates TLR4 in innate immune cells and caspase 1 in subcapsular sinus macrophages, induces differentiation of monocytes to DC, and activates NF-κB and production of IFNγ |
| CpG 1018 (2018) | 22 nucleotide single-stranded DNA containing unmethylated cytosine phospho-guanosine dinucleotide | Hepatitis B | Activates TLR9 resulting in a type I interferon response |
| Viral Vectors (2020) | Adenoviruses carrying mRNA encoding for protein antigen | COVID-19 | PAMPs on the surface of adenoviral carriers activate the innate immune system while also facilitating transfection for mRNA vaccines |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogostin, B.H.; McHugh, K.J. Novel Vaccine Adjuvants as Key Tools for Improving Pandemic Preparedness. Bioengineering 2021, 8, 155. https://doi.org/10.3390/bioengineering8110155
Pogostin BH, McHugh KJ. Novel Vaccine Adjuvants as Key Tools for Improving Pandemic Preparedness. Bioengineering. 2021; 8(11):155. https://doi.org/10.3390/bioengineering8110155
Chicago/Turabian StylePogostin, Brett H., and Kevin J. McHugh. 2021. "Novel Vaccine Adjuvants as Key Tools for Improving Pandemic Preparedness" Bioengineering 8, no. 11: 155. https://doi.org/10.3390/bioengineering8110155
APA StylePogostin, B. H., & McHugh, K. J. (2021). Novel Vaccine Adjuvants as Key Tools for Improving Pandemic Preparedness. Bioengineering, 8(11), 155. https://doi.org/10.3390/bioengineering8110155