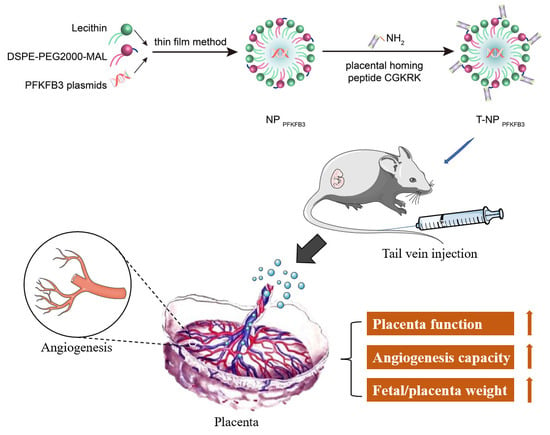
Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Procedures
2.2. Synthesis of Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Nanoparticle Treatment Study
2.5. Immunohistochemistry
2.6. RT-qPCR
2.7. Western Blot Analysis
2.8. Measurements of ALT, AST, and BUN Levels
2.9. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Placenta-Targeted Nanoparticles Loaded with PFKFB3 Plasmids
3.2. T-NPPFKFB3 Selectively Accumulates in the Mouse Placenta and Upregulates PFKFB3 Expression
3.3. T-NPPFKFB3 Promotes Placental Angiogenesis and Increases the Placental and Fetal Weights in Mice
3.4. T-NPPFKFB3 Has No Obvious Side Effects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Liang, R.; Zheng, M.; Cai, L.; Fan, X. Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications. Int. J. Mol. Sci. 2019, 20, 3642. [Google Scholar] [CrossRef] [Green Version]
- Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011, 306, 2459–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charnock, J.C.; Dilworth, M.R.; Aplin, J.D.; Sibley, C.P.; Westwood, M.; Crocker, I.P. The impact of a human IGF-II analog ([Leu27]IGF-II) on fetal growth in a mouse model of fetal growth restriction. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E24–E31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, J.L.; Andersson, I.J.; Poudel, R.; Rueda-Clausen, C.F.; Sibley, C.P.; Davidge, S.T.; Baker, P.N. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension 2012, 59, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisk, N.M.; Atun, R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008, 5, e22. [Google Scholar] [CrossRef] [Green Version]
- Fisk, N.M.; McKee, M.; Atun, R. Relative and absolute addressability of global disease burden in maternal and perinatal health by investment in R&D. Trop. Med. Int. Health 2011, 16, 662–668. [Google Scholar] [CrossRef]
- He, B.; Yang, X.; Li, Y.; Huang, D.; Xu, X.; Yang, W.; Dai, Y.; Zhang, H.; Chen, Z.; Cheng, W. TLR9 (Toll-Like Receptor 9) Agonist Suppresses Angiogenesis by Differentially Regulating VEGFA (Vascular Endothelial Growth Factor A) and sFLT1 (Soluble Vascular Endothelial Growth Factor Receptor 1) in Preeclampsia. Hypertension 2018, 71, 671–680. [Google Scholar] [CrossRef]
- Dakouane-Giudicelli, M.; Brouillet, S.; Traboulsi, W.; Torre, A.; Vallat, G.; Si Nacer, S.; Vallee, M.; Feige, J.J.; Alfaidy, N.; de Mazancourt, P. Inhibition of human placental endothelial cell proliferation and angiogenesis by netrin-4. Placenta 2015, 36, 1260–1265. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial cell metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [Green Version]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquiere, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Schoors, S.; De Bock, K.; Cantelmo, A.R.; Georgiadou, M.; Ghesquiere, B.; Cauwenberghs, S.; Kuchnio, A.; Wong, B.W.; Quaegebeur, A.; Goveia, J.; et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014, 19, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potente, M.; Carmeliet, P. The link between angiogenesis and endothelial metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef]
- Van Schaftingen, E.; Lederer, B.; Bartrons, R.; Hers, H.G. A kinetic study of pyrophosphate: Fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur. J. Biochem. 1982, 129, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Pan, H.; Liu, Z.; Xie, J.; Han, W. Roles of PFKFB3 in cancer. Signal Transduct. Target Ther. 2017, 2, 17044. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Liu, X.; Liu, W.; Zhang, Y.; Wu, M.; Chen, Z.; Zhao, Y.; Zou, L. MALAT1 sponges miR-26a and miR-26b to regulate endothelial cell angiogenesis via PFKFB3-driven glycolysis in early-onset preeclampsia. Mol. Ther. Nucleic Acids 2021, 23, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Zheng, Q.; Bao, C.; He, J.; Chen, B.; Lyu, D.; Zheng, B.; Xu, Y.; Long, Z.; et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017, 388, 208–219. [Google Scholar] [CrossRef]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-based therapeutics and theranostics drug delivery systems for next generation of liver cancer nanodrug modalities. Int. J. Nanomed. 2020, 15, 1437–1456. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.K. Could peptide-decorated nanoparticles provide an improved approach for treating pregnancy complications? Nanomedicine 2016, 11, 2235–2238. [Google Scholar] [CrossRef] [Green Version]
- Irvin-Choy, N.S.; Nelson, K.M.; Gleghorn, J.P.; Day, E.S. Design of nanomaterials for applications in maternal/fetal medicine. J. Mater. Chem. B 2020, 8, 6548–6561. [Google Scholar] [CrossRef]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beards, F.; Jones, L.E.; Charnock, J.; Forbes, K.; Harris, L.K. Placental Homing Peptide-microRNA Inhibitor Conjugates for Targeted Enhancement of Intrinsic Placental Growth Signaling. Theranostics 2017, 7, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, X.; Guo, X.; Habtetsion, T.G.; Wang, Y.; Xu, X.; Kandala, S.; Li, Q.; Li, H.; Zhang, C.; et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1231–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.Y.; Wang, L.; Wang, Y.S.; Dou, G.R. PFKFB3: A potential key to ocular angiogenesis. Front. Cell Dev. Biol. 2021, 9, 628317. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Alanine and aspartate aminotransferase and glutamine-cycling pathway: Their roles in pathogenesis of metabolic syndrome. World J. Gastroenterol. 2012, 18, 3775–3781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.E.; Niu, M.; Li, R.Y.; Feng, W.W.; Ma, X.; Dong, Q.; Ma, Z.J.; Li, G.Q.; Meng, Y.K.; Wang, Y.; et al. Untargeted metabolomics reveals dose-response characteristics for effect of rhubarb in a rat model of cholestasis. Front. Pharmacol. 2016, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Leelahavanichkul, A.; Souza, A.C.; Street, J.M.; Hsu, V.; Tsuji, T.; Doi, K.; Li, L.; Hu, X.; Zhou, H.; Kumar, P.; et al. Comparison of serum creatinine and serum cystatin C as biomarkers to detect sepsis-induced acute kidney injury and to predict mortality in CD-1 mice. Am. J. Physiol. Renal. Physiol. 2014, 307, F939–F948. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zhang, L.; Zhang, Y.; Yu, X.; Sun, X.; Zhu, T.; Li, X.; Liang, W.; Han, Y.; Qin, C. PINK1 deficiency ameliorates cisplatin-induced acute kidney injury in rats. Front. Physiol. 2019, 10, 1225. [Google Scholar] [CrossRef]
- Roset Bahmanyar, E.; Out, H.J.; van Duin, M. Women and babies are dying from inertia: A collaborative framework for obstetrical drug development is urgently needed. Am. J. Obstet. Gynecol. 2021, 225, 43–50. [Google Scholar] [CrossRef]
- Refuerzo, J.S.; Longo, M.; Godin, B. Targeted nanoparticles in pregnancy: A new frontier in perinatal therapeutics. Am. J. Obstet. Gynecol. 2017, 216, 204–205. [Google Scholar] [CrossRef]
- Ali, I.; Alsehli, M.; Scotti, L.; Tullius Scotti, M.; Tsai, S.T.; Yu, R.S.; Hsieh, M.F.; Chen, J.C. Progress in polymeric nano-medicines for theranostic cancer treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftery, R.M.; Tierney, E.G.; Curtin, C.M.; Cryan, S.A.; O’Brien, F.J. Development of a gene-activated scaffold platform for tissue engineering applications using chitosan-pDNA nanoparticles on collagen-based scaffolds. J. Control. Release 2015, 210, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Montemagno, C.; Leary, J.F.; Ritch, R. Regenerative nanomedicine and the treatment of degenerative retinal diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 113–137. [Google Scholar] [CrossRef]
- Campagnolo, L.; Massimiani, M.; Vecchione, L.; Piccirilli, D.; Toschi, N.; Magrini, A.; Bonanno, E.; Scimeca, M.; Castagnozzi, L.; Buonanno, G.; et al. Silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus. Nanotoxicology 2017, 11, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Mottola, F.; Iovine, C.; Santonastaso, M.; Romeo, M.L.; Pacifico, S.; Cobellis, L.; Rocco, L. NPs-TiO2 and lincomycin coexposure induces DNA damage in cultured human amniotic cells. Nanomaterials 2019, 9, 1511. [Google Scholar] [CrossRef] [Green Version]
- Keelan, J.A.; Leong, J.W.; Ho, D.; Iyer, K.S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015, 10, 2229–2247. [Google Scholar] [CrossRef]
- Whigham, C.A.; MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta 2019, 84, 28–31. [Google Scholar] [CrossRef]
- Furth, P.A.; Hennighausen, L.; Baker, C.; Beatty, B.; Woychick, R. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res. 1991, 19, 6205–6208. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.V.; Christoph, G.; Zeller, R.; Leder, P. The cytomegalovirus enhancer: A pan-active control element in transgenic mice. Mol. Cell. Biol. 1990, 10, 4406–4411. [Google Scholar] [CrossRef]
- Arita, E.; Kondoh, M.; Isoda, K.; Nishimori, H.; Yoshida, T.; Mizuguchi, H.; Yagi, K. Evaluation of promoter strength in mouse and rat primary hepatocytes using adenovirus vectors. Eur. J. Pharm. Biopharm. 2008, 70, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, N.; Kaitu’u-Lino, T.; Harris, L.; Tong, S.; Hannan, N. Nanoparticles in pregnancy: The next frontier in reproductive therapeutics. Hum. Reprod. Update 2021, 27, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, M.R.; Sibley, C.P. Review: Transport across the placenta of mice and women. Placenta 2013, 34, S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.P. Treating the dysfunctional placenta. J. Endocrinol. 2017, 234, R81–R97. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, X.; Liu, W.; Zhang, Y.; Liu, W.; Wu, M.; Chen, Z.; Zhao, Y.; Zou, L. Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function. Bioengineering 2022, 9, 652. https://doi.org/10.3390/bioengineering9110652
Li Q, Liu X, Liu W, Zhang Y, Liu W, Wu M, Chen Z, Zhao Y, Zou L. Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function. Bioengineering. 2022; 9(11):652. https://doi.org/10.3390/bioengineering9110652
Chicago/Turabian StyleLi, Qi, Xiaoxia Liu, Weifang Liu, Yang Zhang, Wen Liu, Mengying Wu, Zhirui Chen, Yin Zhao, and Li Zou. 2022. "Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function" Bioengineering 9, no. 11: 652. https://doi.org/10.3390/bioengineering9110652
APA StyleLi, Q., Liu, X., Liu, W., Zhang, Y., Liu, W., Wu, M., Chen, Z., Zhao, Y., & Zou, L. (2022). Placenta-Targeted Nanoparticles Loaded with PFKFB3 Overexpression Plasmids Enhance Angiogenesis and Placental Function. Bioengineering, 9(11), 652. https://doi.org/10.3390/bioengineering9110652