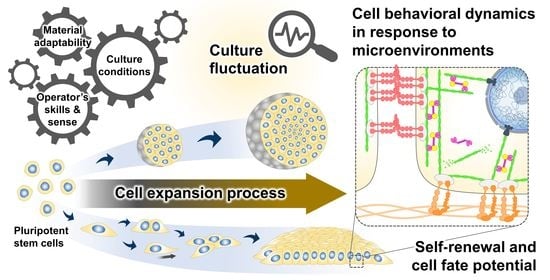
Cell Behavioral Dynamics as a Cue in Optimizing Culture Stabilization in the Bioprocessing of Pluripotent Stem Cells
Abstract
:1. Introduction
2. Mechanistic Roles of Cell Behavioral Dynamics in Modulating Cell Fate Decision
3. Emerging Methods for Enhancing PSC Expansion through the Regulation of Cell Behaviors
| Classification | Tools | References |
|---|---|---|
| Laminin-based culture substrates |
| [53,83,84] |
| [53,83] | |
| Synthetic polymer- and peptide-based culture substrates |
| [85,86,87] |
| [35,79,90,91] | |
| [75] | |
| [88] | |
| E-cadherin-based culture substrates |
| [93,94] |
| E-cadherin function-blocking agents |
| [67,81,95] |
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.W.; Warnock, G.L.; Levings, M.K.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061.e5. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef]
- Wang, Y.; Chou, B.K.; Dowey, S.; He, C.; Gerecht, S.; Cheng, L. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res. 2013, 11, 1103–1116. [Google Scholar] [CrossRef] [Green Version]
- Chen, V.C.; Ye, J.; Shukla, P.; Hua, G.; Chen, D.; Lin, Z.; Liu, J.C.; Chai, J.; Gold, J.; Wu, J.; et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015, 15, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi-Aoi, M.; Ohnuki, M.; Takahashi, K.; Okita, K.; Noma, H.; Sawamura, Y.; Teramoto, I.; Narita, M.; Sato, Y.; Ichisaka, T.; et al. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20569–20574. [Google Scholar] [CrossRef] [Green Version]
- Cahan, P.; Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 2013, 14, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokhale, P.J.; Au-Young, J.K.; Dadi, S.V.; Keys, D.N.; Harrison, N.J.; Jones, M.; Soneji, S.; Enver, T.; Sherlock, J.K.; Andrews, P.W. Culture adaptation alters transcriptional hierarchies among single human embryonic stem cells reflecting altered patterns of differentiation. PLoS ONE 2015, 10, e0123467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phadnis, S.M.; Loewke, N.O.; Dimov, I.K.; Pai, S.; Amwake, C.E.; Solgaard, O.; Baer, T.M.; Chen, B.; Pera, R.A.R. Dynamic and social behaviors of human pluripotent stem cells. Sci. Rep. 2015, 5, 14209. [Google Scholar] [CrossRef]
- Paniza, T.; Deshpande, M.; Wang, N.; Neil, O.R.; Zuccaro, M.V.; Smith, M.E.; Madireddy, A.; James, D.; Ecker, J.; Rosenwaks, Z.; et al. Pluripotent stem cells with low differentiation potential contain incompletely reprogrammed DNA replication. J. Cell Biol. 2020, 219, e201909163. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Human induced pluripotent stem cells: From cell origin, genomic stability, and epigenetic memory to translational medicine. Stem Cells 2022, 40, 546–555. [Google Scholar] [CrossRef]
- Chowdhury, F.; Na, S.; Li, D.; Poh, Y.C.; Tanaka, T.S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2009, 9, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kino-oka, M. Bioengineering considerations for a nurturing way to enhance scalable expansion of human pluripotent stem cells. Biotechnol. J. 2020, 15, 1900314. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fan, Y.; Guo, Z.; Wang, Y.; Zheng, X.; Huang, C.; Liang, B.; Gao, L.; Cao, Y.; Chen, Y.; et al. Compression generated by a 3D supracellular actomyosin cortex promotes embryonic stem cell colony growth and expression of Nanog and Oct4. Cell Syst. 2019, 9, 214–220.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Li, J.; Hong, J.; Takashima, Y.; Fujimoto, N.; Nakajima, M.; Yamamoto, A.; Dong, X.; Dang, Y.; Hou, Y.; et al. Low Cell-matrix adhesion reveals two subtypes of human pluripotent stem cells. Stem Cell Rep. 2018, 11, 142–156. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.; Seo, K.; Kim, J.H.; Lee, B.; Cho, H.M.; Kim, H.J.; Yang, E.; Kim, H.; Gim, J.A.; et al. Cell position within human pluripotent stem cell colonies determines apical specialization via an actin cytoskeleton-based mechanism. Stem Cell Rep. 2022, 17, 68–81. [Google Scholar] [CrossRef]
- Rosowski, K.A.; Mertz, A.F.; Norcross, S.; Dufresne, E.R.; Horsley, V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci. Rep. 2015, 5, 14218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorman, B.R.; Lu, J.; Baccei, A.; Lowry, N.C.; Purvis, J.E.; Mangoubi, R.S.; Lerou, P.H. Multi-scale imaging and informatics pipeline for in situ pluripotent stem cell analysis. PLoS ONE 2014, 9, e116037. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H.; Thanuthanakhun, N.; Fujimoto, S.; Kino-oka, M. Effect of initial seeding density on cell behavior-driven epigenetic memory and preferential lineage differentiation of human iPSCs. Stem Cell Res. 2021, 56, 102534. [Google Scholar] [CrossRef]
- Kim, M.H.; Takeuchi, K.; Kino-oka, M. Role of cell-secreted extracellular matrix formation in aggregate formation and stability of human induced pluripotent stem cells in suspension culture. J. Biosci. Bioeng. 2019, 127, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kim, M.H.; Kino-oka, M. Comparison of growth kinetics between static and dynamic cultures of human induced pluripotent stem cells. J. Biosci. Bioeng. 2018, 125, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Hashida, A.; Uemura, T.; Kino-oka, M. Kinetics on aggregate behaviors of human induced pluripotent stem cells in static suspension and rotating flow cultures. J. Biosci. Bioeng. 2020, 129, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Thanuthanakhun, N.; Kino-oka, M.; Borwornpinyo, S.; Ito, Y.; Kim, M.H. The impact of culture dimensionality on behavioral epigenetic memory contributing to pluripotent state of iPS cells. J. Cell. Physiol. 2021, 236, 4985–4996. [Google Scholar] [CrossRef]
- Keong Kwok, C.; Sébastien, I.; Hariharan, K.; Meiser, I.; Wihan, J.; Altmaier, S.; Karnatz, I.; Feile, A.; Cabrera-Socorro, A.; Rasmussen, M.; et al. Scalable expansion of iPSC and their derivatives across multiple lineages. Reprod. Toxicol. 2022, 112, 23–35. [Google Scholar] [CrossRef]
- Weissbein, U.; Plotnik, O.; Vershkov, D.; Benvenisty, N. Culture-induced recurrent epigenetic aberrations in human pluripotent stem cells. PLoS Genet. 2017, 13, e1006979. [Google Scholar] [CrossRef] [Green Version]
- David, B.G.; Fujita, H.; Yasuda, K.; Okamoto, K.; Panina, Y.; Ichinose, J.; Sato, O.; Horie, M.; Ichimura, T.; Okada, Y.; et al. Linking substrate and nucleus via actin cytoskeleton in pluripotency maintenance of mouse embryonic stem cells. Stem Cell Res. 2019, 41, 101614. [Google Scholar] [CrossRef]
- Kim, I.G.; Gil, C.H.; Seo, J.; Park, S.J.; Subbiah, R.; Jung, T.H.; Kim, J.S.; Jeong, Y.H.; Chung, H.M.; Lee, J.H.; et al. Mechanotransduction of human pluripotent stem cells cultivated on tunable cell-derived extracellular matrix. Biomaterials 2018, 150, 100–111. [Google Scholar] [CrossRef]
- Bauwens, C.L.; Peerani, R.; Niebruegge, S.; Woodhouse, K.A.; Kumacheva, E.; Husain, M.; Zandstra, P.W. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 2008, 26, 2300–2310. [Google Scholar] [CrossRef]
- Naqvi, S.M.; McNamara, L.M. Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661. [Google Scholar] [CrossRef]
- Toh, Y.C.; Xing, J.; Yu, H. Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation. Biomaterials 2015, 50, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rico, C.; Pincet, F.; Thiery, J.P.; Dufour, S. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J. Cell Sci. 2010, 123, 712–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, D.; Chen, S.C.; Prasad, M.; He, L.; Wang, X.; Choesmel-Cadamuro, V.; Sawyer, J.K.; Danuser, G.; Montell, D.J. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 2014, 157, 1146–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca-Cusachs, P.; Gauthier, N.C.; Del Rio, A.; Sheetz, M.P. Clustering of A5β1 integrins determines adhesion strength whereas Avβ3 and talin enable mechanotransduction. Proc. Natl. Acad. Sci. USA 2009, 106, 16245–16250. [Google Scholar] [CrossRef] [Green Version]
- Labouesse, C.; Tan, B.X.; Agley, C.C.; Hofer, M.; Winkel, A.K.; Stirparo, G.G.; Stuart, H.T.; Verstreken, C.M.; Mulas, C.; Mansfield, W.; et al. StemBond hydrogels control the mechanical microenvironment for pluripotent stem cells. Nat. Commun. 2021, 12, 6132. [Google Scholar] [CrossRef]
- Gupton, S.L.; Waterman-Storer, C.M. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 2006, 125, 1361–1374. [Google Scholar] [CrossRef] [Green Version]
- Ciobanasu, C.; Faivre, B.; Le Clainche, C. Actomyosin-dependent formation of the mechanosensitive talin–vinculin complex reinforces actin anchoring. Nat. Commun. 2014, 5, 3095. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhu, X.; Hahm, H.S.; Wei, W.; Hao, E.; Hayek, A.; Ding, S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl. Acad. Sci. USA 2010, 107, 8129–8134. [Google Scholar] [CrossRef] [Green Version]
- Närvä, E.; Stubb, A.; Guzmán, C.; Blomqvist, M.; Balboa, D.; Lerche, M.; Saari, M.; Otonkoski, T.; Ivaska, J. A strong contractile actin fence and large adhesions direct human pluripotent colony morphology and adhesion. Stem Cell Rep. 2017, 9, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Harb, N.; Archer, T.K.; Sato, N. The Rho-Rock-myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS ONE 2008, 3, e3001. [Google Scholar] [CrossRef]
- Chen, G.; Hou, Z.; Gulbranson, D.R.; Thomson, J.A. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 2010, 7, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.K.; Ishihara, T.; Tanaka, H.; Ishijima, A.; Inoue, Y. Velocity-dependent actomyosin ATPase cycle revealed by in vitro motility assay with kinetic analysis. Biophys. J. 2012, 103, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noren, N.K.; Liu, B.P.; Burridge, K.; Kreft, B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 2000, 150, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.G.; Zhang, Q.; Prasad, N.; Li, Y.; Chamala, S.; Kuchibhotla, R.; Kc, B.; Aggarwal, V.; Shrestha, S.; Jones, A.L.; et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 2016, 6, 38063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alisafaei, F.; Jokhun, D.S.; Shivashankar, G.V.; Shenoy, V.B. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proc. Natl. Acad. Sci. USA 2019, 116, 13200–13209. [Google Scholar] [CrossRef] [Green Version]
- Grespan, E.; Giobbe, G.G.; Badique, F.; Anselme, K.; Rühe, J.; Elvassore, N. Effect of geometrical constraints on human pluripotent stem cell nuclei in pluripotency and differentiation. Integr. Biol. 2018, 10, 278–289. [Google Scholar] [CrossRef]
- Chi, Y.H.; Wang, W.P.; Hung, M.C.; Liou, G.G.; Wang, J.Y.; Chao, P.H.G. Deformation of the nucleus by TGFβ1 via the remodeling of nuclear envelope and histone isoforms. Epigenetics Chromatin 2022, 15, 1. [Google Scholar] [CrossRef]
- Damodaran, K.; Venkatachalapathy, S.; Alisafaei, F.; Radhakrishnan, A.V.; Jokhun, D.S.; Shenoy, V.B.; Shivashankar, G.V. Compressive force induces reversible chromatin condensation and cell geometry–dependent transcriptional response. Mol. Biol. Cell 2018, 29, 3039–3051. [Google Scholar] [CrossRef]
- Jain, N.; Iyer, K.V.; Kumar, A.; Shivashankar, G.V. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc. Natl. Acad. Sci. USA 2013, 110, 11349–11354. [Google Scholar] [CrossRef] [Green Version]
- Vite, A.; Zhang, C.; Yi, R.; Emms, S.; Radice, G.L. α-catenin-dependent cytoskeletal tension controls Yap activity in the heart. Development 2018, 145, dev149823. [Google Scholar] [CrossRef]
- Furukawa, K.T.; Yamashita, K.; Sakurai, N.; Ohno, S. The epithelial circumferential actin belt regulates YAP/TAZ through nucleocytoplasmic shuttling of Merlin. Cell Rep. 2017, 20, 1435–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohgushi, M.; Minaguchi, M.; Sasai, Y. Rho-signaling-directed YAP/TAZ activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell 2015, 17, 448–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, S.; Hayashi, R.; Okubo, T.; Kudo, Y.; Katayama, T.; Ishikawa, Y.; Toga, J.; Yagi, E.; Honma, Y.; Quantock, A.J.; et al. Selective laminin-directed differentiation of human induced pluripotent stem cells into distinct ocular lineages. Cell Rep. 2018, 25, 1668–1679.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.B.; Abujarour, R.; et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passaro, F.; de Martino, I.; Zambelli, F.; Di Benedetto, G.; Barbato, M.; D’Erchia, A.M.; Manzari, C.; Pesole, G.; Mutarelli, M.; Cacchiarelli, D.; et al. YAP contributes to DNA methylation remodeling upon mouse embryonic stem cell differentiation. J. Biol. Chem. 2021, 296, 100138. [Google Scholar] [CrossRef]
- Sun, X.; Ren, Z.; Cun, Y.; Zhao, C.; Huang, X.; Zhou, J.; Hu, R.; Su, X.; Ji, L.; Li, P.; et al. Hippo-YAP signaling controls lineage differentiation of mouse embryonic stem cells through modulating the formation of super-enhancers. Nucleic Acids Res. 2020, 48, 7182–7196. [Google Scholar] [CrossRef]
- Zhou, X.; Chadarevian, J.P.; Ruiz, B.; Ying, Q.L. Cytoplasmic and nuclear TAZ exert distinct functions in regulating primed pluripotency. Stem Cell Rep. 2017, 9, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Robitaille, A.M.; Berndt, J.D.; Davidson, K.C.; Fischer, K.A.; Mathieu, J.; Potter, J.C.; Ruohola-Baker, H.; Moon, R.T. Wnt/β-Catenin signaling promotes self-renewal and inhibits the primed state transition in naïve human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6382–E6390. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Go, Y.; Kang, I.; Han, Y.M.; Kim, J. Oct-4 controls cell-cycle progression of embryonic stem cells. Biochem. J. 2010, 426, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Adachi, K.; Suemori, H.; Yasuda, S.Y.; Nakatsuji, N.; Kawase, E. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells 2010, 15, 455–469. [Google Scholar]
- Przybyla, L.; Lakins, J.N.; Weaver, V.M. Tissue mechanics orchestrate Wnt-Dependent human embryonic stem cell differentiation. Cell Stem Cell 2016, 19, 462–475. [Google Scholar] [CrossRef]
- Cattavarayane, S.; Palovuori, R.; Tanjore Ramanathan, J.; Manninen, A. A6β1- and AV-integrins are required for long-term self-renewal of murine embryonic stem cells in the absence of LIF. BMC Cell Biol. 2015, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitillo, L.; Baxter, M.; Iskender, B.; Whiting, P.; Kimber, S.J. Integrin-associated focal adhesion kinase protects human embryonic stem cells from apoptosis, detachment, and differentiation. Stem Cell Rep. 2016, 7, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redmer, T.; Diecke, S.; Grigoryan, T.; Quiroga-Negreira, A.; Birchmeier, W.; Besser, D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011, 12, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandy, R.A.; Whitfield, T.W.; Wu, H.; Fitzgerald, M.P.; VanOudenhove, J.J.; Zaidi, S.K.; Montecino, M.A.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L.; et al. Genome-wide studies reveal that H3K4me3 modification in bivalent genes is dynamically regulated during the pluripotent cell cycle and stabilized upon differentiation. Mol. Cell Biol. 2016, 36, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Elsafi Mabrouk, M.H.; Goetzke, R.; Abagnale, G.; Yesilyurt, B.; Salz, L.; Cypris, O.; Glück, P.; Liesenfelder, S.; Zeevaert, K.; Ma, Z.; et al. The spatial self-organization within pluripotent stem cell colonies is continued in detaching aggregates. Biomaterials 2022, 282, 121389. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Sugawara, Y.; Fujinaga, Y.; Kino-Oka, M. Botulinum hemagglutinin-mediated selective removal of cells deviating from the undifferentiated state in hiPSC Colonies. Sci. Rep. 2017, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuzui, E.; Kim, M.H.; Kino-oka, M. Anomalous cell migration triggers a switch to deviation from the undifferentiated state in colonies of human induced pluripotent stems on feeder layers. J. Biosci. Bioeng. 2019, 127, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Sun, Y.; Resto-Irizarry, A.M.; Yuan, Y.; Aw Yong, K.M.; Zheng, Y.; Weng, S.; Shao, Y.; Chai, Y.; Studer, L.; et al. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater. 2018, 17, 633–641. [Google Scholar] [CrossRef]
- Etoc, F.; Metzger, J.; Ruzo, A.; Kirst, C.; Yoney, A.; Ozair, M.Z.; Brivanlou, A.H.; Siggia, E.D. A Balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev. Cell 2016, 39, 302–315. [Google Scholar] [CrossRef] [Green Version]
- Martyn, I.; Brivanlou, A.H.; Siggia, E.D. A wave of WNT signaling balanced by secreted inhibitors controls primitive streak formation in micropattern colonies of human embryonic stem cells. Dev. 2019, 146, dev172791. [Google Scholar] [CrossRef] [PubMed]
- Azarin, S.M.; Lian, X.; Larson, E.A.; Popelka, H.M.; de Pablo, J.J.; Palecek, S.P. Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3-d microwell array. Biomaterials 2012, 33, 2041–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torizal, F.G.; Kim, S.M.; Horiguchi, I.; Inamura, K.; Suzuki, I.; Morimura, T.; Nishikawa, M.; Sakai, Y. Production of homogenous size-controlled human induced pluripotent stem cell aggregates using ring-shaped culture vessel. J. Tissue Eng. Regen. Med. 2022, 16, 254–266. [Google Scholar] [CrossRef]
- McKee, C.; Brown, C.; Chaudhry, G.R. Self-assembling scaffolds supported long-term growth of human primed embryonic stem cells and upregulated core and naïve pluripotent markers. Cells 2019, 8, 1650. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.H.; Chao, H.M.; Chern, E.; Hsu, S. Chitosan 3D cell culture system promotes naïve-like features of human induced pluripotent stem cells: A novel tool to sustain pluripotency and facilitate differentiation. Biomaterials 2021, 268, 120575. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.A.; Cotovio, J.P.; Rodrigues, C.A.V.; Vaz, S.H.; Fernandes, T.G.; Moreira, L.M.; Cabral, J.M.S.; Diogo, M.M. Transcriptomic analysis of 3D cardiac differentiation of human induced pluripotent stem cells reveals faster cardiomyocyte maturation compared to 2D culture. Sci. Rep. 2019, 9, 9229. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ye, K. Three-dimensional differentiation of embryonic stem cells into islet-like insulin-producing clusters. Tissue Eng Part A. 2009, 15, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.W.; Binder, B.Y.K.; Khalil, A.S.; Schmitt, S.K.; Johnson, H.J.; Zacharias, N.A.; Murphy, W.L. Controlled self-assembly of stem cell aggregates instructs pluripotency and lineage bias. Sci. Rep. 2017, 7, 14070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, S.; Joanne, P.; Migdal, C.; Soler, E.L.; Hovhannisyan, Y.; Nicolas, A.; Agbulut, O. Polyacrylamide hydrogels with rigidity-independent surface chemistry show limited long-term maintenance of pluripotency of human induced pluripotent stem cells on soft substrates. ACS Biomater. Sci. Eng. 2020, 6, 340–351. [Google Scholar] [CrossRef]
- Panda, A.K.; Ravikumar, R.; Gebrekrstos, A.; Bose, S.; Markandeya, Y.S.; Mehta, B.; Basu, B. Tunable substrate functionalities direct stem cell fate toward electrophysiologically distinguishable neuron-like and glial-like cells. ACS Appl. Mater. Interfaces 2021, 13, 164–185. [Google Scholar] [CrossRef]
- Shuzui, E.; Kim, M.H.; Azuma, K.; Fujinaga, Y.; Kino-oka, M. Maintenance of an undifferentiated state of human-induced pluripotent stem cells through botulinum hemagglutinin-mediated regulation of cell behavior. J. Biosci. Bioeng. 2019, 127, 744–751. [Google Scholar] [CrossRef]
- Lee, S.; Stanton, A.E.; Tong, X.; Yang, F. Hydrogels with enhanced protein conjugation efficiency reveal stiffness-induced YAP localization in stem cells depends on biochemical cues. Biomaterials 2019, 202, 26–34. [Google Scholar] [CrossRef]
- Miyazaki, T.; Futaki, S.; Suemori, H.; Taniguchi, Y.; Yamada, M.; Kawasaki, M.; Hayashi, M.; Kumagai, H.; Nakatsuji, N.; Sekiguchi, K.; et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012, 3, 1236. [Google Scholar] [CrossRef] [Green Version]
- Laperle, A.; Hsiao, C.; Lampe, M.; Mortier, J.; Saha, K.; Palecek, S.P.; Masters, K.S. α-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Rep. 2015, 5, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Brafman, D.A.; Chang, C.W.; Fernandez, A.; Willert, K.; Varghese, S.; Chien, S. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 2010, 31, 9135–9144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Villa-Diaz, L.G.; Kumar, R.; Lahann, J.; Krebsbach, P.H. Enhancement of the propagation of human embryonic stem cells by modifications in the gel architecture of PMEDSAH polymer coatings. Biomaterials 2014, 35, 9581–9590. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, E.; Iguchi, H.; Le, M.N.; Nakamura, Y.; Kobayashi, D.; Arai, Y.; Takakura, K.; Benno, S.; Yoshida, N.; Tsukahara, M.; et al. A chemically-defined plastic scaffold for the xeno-free production of human pluripotent stem cells. Sci. Rep. 2022, 12, 2516. [Google Scholar] [CrossRef]
- Sung, T.C.; Li, H.F.; Higuchi, A.; Kumar, S.S.; Ling, Q.D.; Wu, Y.W.; Burnouf, T.; Nasu, M.; Umezawa, A.; Lee, K.F.; et al. Effect of cell culture biomaterials for completely xeno-free generation of human induced pluripotent stem cells. Biomaterials 2020, 230, 119638. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zu, Y.; Li, J.; Du, S.; Xu, Y.; Zhang, L.; Jiang, L.; Wang, Z.; Chien, S.; Yang, C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci. Rep. 2016, 6, 20395. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, A.; Kao, S.H.; Ling, Q.D.; Chen, Y.M.; Li, H.F.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Chang, S.C.; Lee, H.C.; et al. Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity. Sci. Rep. 2015, 5, 18136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, J.E.; Shah, D.A.; Rogers, C.; Hall, S.; Weston, N.; Parmenter, C.D.J.; McNally, D.; Denning, C.; Shakesheff, K.M. Combined hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Aban, C.E.; Lombardi, A.; Neiman, G.; Biani, M.C.; La Greca, A.; Waisman, A.; Moro, L.N.; Sevlever, G.; Miriuka, S.; Luzzani, C. Downregulation of E-Cadherin in pluripotent stem cells triggers partial EMT. Sci. Rep. 2021, 11, 2048. [Google Scholar] [CrossRef]
- Nagaoka, M.; Koshimizu, U.; Yuasa, S.; Hattori, F.; Chen, H.; Tanaka, T.; Okabe, M.; Fukuda, K.; Akaike, T. E-cadherin-coated plates maintain pluripotent es cells without colony formation. PLoS ONE 2006, 1, e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaoka, M.; Duncan, S.A. Laboratory-scale purification of a recombinant E-cadherin-IgG Fc fusion protein that provides a cell surface matrix for extended culture and efficient subculture of human pluripotent stem cells. In Human Embryonic and Induced Pluripotent Stem Cells; Ye, K., Jin, S., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 25–35. ISBN 978-1-61779-267-0. [Google Scholar]
- Nath, S.C.; Tokura, T.; Kim, M.H.; Kino-oka, M. Botulinum hemagglutinin-mediated in situ break-up of human induced pluripotent stem cell aggregates for high-density suspension culture. Biotechnol. Bioeng. 2018, 115, 910–920. [Google Scholar] [CrossRef]
- Creighton, H.; Waddington, C.H. The strategy of the genes. AIBS Bull. 1958, 8, 49. [Google Scholar] [CrossRef]
- Hsiao, C.; Lampe, M.; Nillasithanukroh, S.; Han, W.; Lian, X.; Palecek, S.P. Human Pluripotent stem cell culture density modulates YAP signaling. Biotechnol. J. 2016, 11, 662–675. [Google Scholar] [CrossRef] [Green Version]
- Kashkooli, L.; Rozema, D.; Espejo-Ramirez, L.; Lasko, P.; Fagotto, F. Ectoderm to mesoderm transition by down-regulation of actomyosin contractility. PLoS Biol. 2021, 9, e3001060. [Google Scholar] [CrossRef]
- Velazquez, J.J.; Legraw, R.; Moghadam, F.; Tan, Y.; Kilbourne, J.; Maggiore, J.C.; Hislop, J.; Liu, S.; Cats, D.; Chuva de Sousa Lopes, S.M.; et al. Gene regulatory network analysis and engineering directs development and vascularization of multilineage human liver organoids. Cell Syst. 2021, 12, 41–55.e11. [Google Scholar] [CrossRef]
- Sladitschek, H.L.; Neveu, P.A. A gene regulatory network controls the balance between mesendoderm and ectoderm at pluripotency exit. Mol. Syst. Biol. 2019, 15, e9043. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Luu, R.J.; Ramos, M.E.P.; Nam, J. ROCK inhibitor primes human induced pluripotent stem cells to selectively differentiate towards mesendodermal lineage via epithelial-mesenchymal transition-like modulation. Stem. Cell Res. 2016, 17, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.M. Mechanical control of cell differentiation: Insights from the early embryo. Annu. Rev. Biomed. Eng. 2022, 24, 307–322. [Google Scholar] [CrossRef]
- Taylor-Weiner, H.; Ravi, N.; Engler, A.J. Traction forces mediated by integrin signaling are necessary for definitive endoderm specification. J. Cell Sci. 2015, 128, 1961–1968. [Google Scholar] [CrossRef] [Green Version]
- Boraas, L.C.; Pineda, E.T.; Ahsan, T. Actin and myosin II modulate differentiation of pluripotent stem cells. PLoS ONE 2018, 13, e0195588. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.G.; Alsolami, S.M.; Arossa, S.; Ramos-Mandujano, G.; Parry, A.J.; Steckbauer, A.; Duarte, C.M.; Li, M. In situ monitoring reveals cellular environmental instabilities in human pluripotent stem cell culture. Commun. Biol. 2022, 5, 119. [Google Scholar] [CrossRef]
- Quintanilla, R.H., Jr.; Asprer, J.S.T.; Vaz, C.; Tanavde, V.; Lakshmipathy, U. CD44 is a negative cell surface marker for pluripotent stem cell identification during human fibroblast reprogramming. PLoS ONE 2014, 9, e85419. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, A.S.E.; Seaton, D.D.; McCarthy, D.J.; Martinez, I.; Bonder, M.J.; Garcia-Bernardo, J.; Amatya, S.; Madrigal, P.; Isaacson, A.; Buettner, F.; et al. Single-cell RNA-sequencing of differentiating iPS cells reveals dynamic genetic effects on gene expression. Nat. Commun. 2020, 11, 810. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.C.; Xu, J.; Brinkhof, B.; Wang, H.; Cui, Z.; Huang, W.E.; Ye, H. A single-cell Raman-based platform to identify developmental stages of human pluripotent stem cell-derived neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 18412–18423. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanuthanakhun, N.; Kim, M.-H.; Kino-oka, M. Cell Behavioral Dynamics as a Cue in Optimizing Culture Stabilization in the Bioprocessing of Pluripotent Stem Cells. Bioengineering 2022, 9, 669. https://doi.org/10.3390/bioengineering9110669
Thanuthanakhun N, Kim M-H, Kino-oka M. Cell Behavioral Dynamics as a Cue in Optimizing Culture Stabilization in the Bioprocessing of Pluripotent Stem Cells. Bioengineering. 2022; 9(11):669. https://doi.org/10.3390/bioengineering9110669
Chicago/Turabian StyleThanuthanakhun, Naruchit, Mee-Hae Kim, and Masahiro Kino-oka. 2022. "Cell Behavioral Dynamics as a Cue in Optimizing Culture Stabilization in the Bioprocessing of Pluripotent Stem Cells" Bioengineering 9, no. 11: 669. https://doi.org/10.3390/bioengineering9110669
APA StyleThanuthanakhun, N., Kim, M. -H., & Kino-oka, M. (2022). Cell Behavioral Dynamics as a Cue in Optimizing Culture Stabilization in the Bioprocessing of Pluripotent Stem Cells. Bioengineering, 9(11), 669. https://doi.org/10.3390/bioengineering9110669