Polymeric Materials as Indispensable Tools to Fight RNA Viruses: SARS-CoV-2 and Influenza A
Abstract
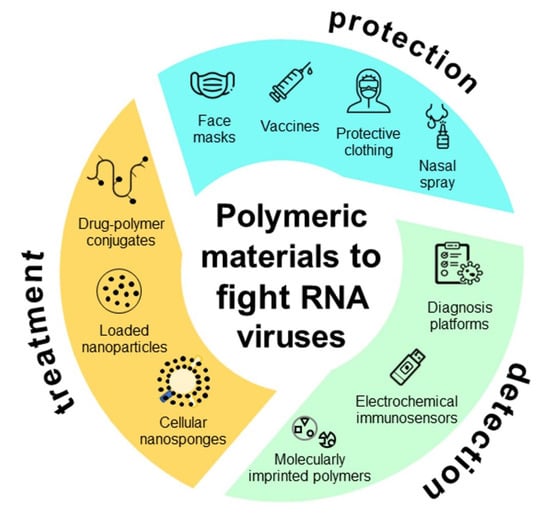
:1. Introduction
Flu or Corona? Maybe Flurona
2. Protection
3. Detection
4. Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almaghaslah, D.; Kandasamy, G.; Almanasef, M.; Vasudevan, R.; Chandramohan, S. Review on the Coronavirus Disease (COVID-19) Pandemic: Its Outbreak and Current Status. Int. J. Clin. Pract. 2020, 74, e13637. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Lu, C.C.; Bau, D.T.; Chiu, Y.J.; Yen, Y.T.; Hsu, Y.M.; Fu, C.W.; Kuo, S.C.; Lo, Y.S.; Chiu, H.Y.; et al. Approaches towards Fighting the COVID-19 Pandemic (Review). Int. J. Mol. Med. 2021, 47, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Medicine, A. Past, Present and Future of Covid-19 Pandemic; Review of the Pathophysiology and Clinical Management. Ann. Clin. Anal. Med. 2021, 12, 822–828. [Google Scholar]
- Sadeghi Dousari, A.; Taati Moghadam, M.; Satarzadeh, N. COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease. Infect. Drug Resist. 2020, 13, 2819–2828. [Google Scholar] [CrossRef]
- Satarker, S.; Nampoothiri, M. Structural Proteins in Severe Acute Respiratory Syndrome Coronavirus-2. Arch. Med. Res. 2020, 51, 482–491. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets for SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, R.; Zhou, Q. ACE2, B0AT1, and SARS-CoV-2 spike protein: Structural and functional implications. Curr. Opin. Struct. Biol. 2022, 74, 102388. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Pizzato, M.; Baraldi, C.; Boscato Sopetto, G.; Finozzi, D.; Gentile, C.; Gentile, M.D.; Marconi, R.; Paladino, D.; Raoss, A.; Riedmiller, I.; et al. SARS-CoV-2 and the Host Cell: A Tale of Interactions. Front. Virol. 2022, 1, 815388. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 2309. [Google Scholar] [CrossRef]
- Bai, Y.; Tao, X. Comparison of COVID-19 and Influenza Characteristics. J. Zhejiang Univ. Sci. B 2021, 22, 87–98. [Google Scholar] [CrossRef]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza Virus and SARS-CoV-2: Pathogenesis and Host Responses in the Respiratory Tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef]
- Mcauley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza Virus Neuraminidase Structure and Functions. Front. Microbiol. 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Manzanares-Meza, L.D.; Medina-Contreras, O. SARS-CoV-2 and Influenza: A Comparative Overview and Treatment Implications. Bol. Med. Hosp. Infant. Mex. 2020, 77, 262–273. [Google Scholar] [CrossRef]
- Kosik, I.; Yewdell, J.W. Influenza Hemagglutinin and Neuraminidase: Yin–Yang Proteins Coevolving to Thwart Immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef] [Green Version]
- Daemi, H.B.; Kulyar, M.F.E.A.; He, X.; Li, C.; Karimpour, M.; Sun, X.; Zou, Z.; Jin, M. Progression and Trends in Virus from Influenza A to Covid-19: An Overview of Recent Studies. Viruses 2021, 13, 1145. [Google Scholar] [CrossRef]
- CDC. Similarities and Differences between Flu and COVID-19; CDC: Atlanta, GA, USA, 2022; pp. 1–5. Available online: https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm#print (accessed on 11 November 2022).
- Khorramdelazad, H.; Kazemi, M.H.; Najafi, A.; Keykhaee, M.; Zolfaghari Emameh, R.; Falak, R. Immunopathological Similarities between COVID-19 and Influenza: Investigating the Consequences of Co-Infection. Microb. Pathog. 2021, 152, 104554. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science. 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 Virus by Droplets and Aerosols: A Critical Review on the Unresolved Dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- CerTest BioTec. Flu A, Flu B & SARS-CoV-2; CerTest BioTec: San Mateo de Gállego, Spain, 2019. [Google Scholar]
- Dadashi, M.; Khaleghnejad, S.; Abedi Elkhichi, P.; Goudarzi, M.; Goudarzi, H.; Taghavi, A.; Vaezjalali, M.; Hajikhani, B. COVID-19 and Influenza Co-Infection: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Alosaimi, B.; Naeem, A.; Hamed, M.E.; Alkadi, H.S.; Alanazi, T.; Al Rehily, S.S.; Almutairi, A.Z.; Zafar, A. Influenza Co-Infection Associated with Severity and Mortality in COVID-19 Patients. Virol. J. 2021, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.; Silvert, E.; Horo, J.C.O.; Lenehan, P.J.; Challener, D.; Gnass, E.; Murugadoss, K.; Ross, J.; Speicher, L.; Geyer, H.; et al. SARS-CoV-2 and Influenza Co-Infection throughout the COVID-19 Pandemic: An Assessment of Co-Infection Rates and Cohort Characterization. medRxiv 2022, 270324. [Google Scholar] [CrossRef]
- Eftekhari, A.; Alipour, M.; Chodari, L.; Dizaj, S.M.; Ardalan, M.R.; Samiei, M.; Sharifi, S.; Vahed, S.Z.; Huseynova, I.; Khalilov, R.; et al. A Comprehensive Review of Detection Methods for SARS-CoV-2. Microorganisms 2021, 9, 232. [Google Scholar] [CrossRef]
- Rong, G.; Zheng, Y.; Chen, Y.; Zhang, Y.; Zhu, P.; Sawan, M. COVID-19 Diagnostic Methods and Detection Techniques: A Review. Ref. Modul. Biomed. Sci. 2021, 3, 17–32. [Google Scholar]
- Han, S.; Ko, O.; Lee, G.; Jeong, S.W.; Choi, Y.J.; Lee, J.B. Rapid Diagnosis of Coronavirus by RNA-Directed RNA Transcription Using an Engineered RNA-Based Platform. Nano Lett. 2021, 21, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.S.; Zambon, M.C. Molecular Diagnosis of Influenza. Rev. Med. Virol. 2002, 12, 375–389. [Google Scholar] [CrossRef]
- Aghbash, P.S.; Eslami, N.; Shirvaliloo, M.; Baghi, H.B. Viral Coinfections in COVID-19. J. Med. Virol. 2021, 93, 5310–5322. [Google Scholar] [CrossRef]
- Rana, M.M. Polymer-Based Nano-Therapies to Combat COVID-19 Related Respiratory Injury: Progress, Prospects, and Challenges. J. Biomater. Sci. Polym. Ed. 2021, 32, 1219–1249. [Google Scholar] [CrossRef]
- Madurani, K.A.; Suprapto, S.; Syahputra, M.Y.; Puspita, I.; Masudi, A.; Rizqi, H.D.; Hatta, A.M.; Juniastuti, J.; Lusida, M.I.; Kurniawan, F. Review—Recent Development of Detection Methods for Controlling COVID-19 Outbreak. J. Electrochem. Soc. 2021, 168, 037511. [Google Scholar] [CrossRef]
- CDC. COVID-19 Treatments and Medications; CDC: Atlanta, GA, USA, 2022; p. 1. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html#print (accessed on 11 November 2022).
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Opu, R.R.; Ahmed, N.; Talukder, A.; Nunia, R.; Chowdhury, M.S.; Nodi, I.J.; Saha, T.; et al. Protective Measures Are Associated with the Reduction of Transmission of COVID-19 in Bangladesh: A Nationwide Cross-Sectional Study. PLoS ONE 2021, 16, e0260287. [Google Scholar] [CrossRef] [PubMed]
- WHO. Technical Specifications of Personal Protective Equipment for COVID-19: Interim Guidance; World Health Organization: Geneve, Switzerland, 2020. [Google Scholar]
- Armentano, I.; Barbanera, M.; Carota, E.; Crognale, S.; Marconi, M.; Rossi, S.; Rubino, G.; Scungio, M.; Taborri, J.; Calabrò, G. Polymer Materials for Respiratory Protection: Processing, End Use, and Testing Methods. ACS Appl. Polym. Mater. 2021, 3, 531–548. [Google Scholar] [CrossRef]
- Sim, S.W.; Moey, K.S.P.; Tan, N.C. The Use of Facemasks to Prevent Respiratory Infection: A Literature Review in the Context of the Health Belief Model. Singapore Med. J. 2014, 55, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, M.; Liu, H.; Wang, X.; Hu, X.; Huang, Y.; Liu, X.; Brenan, K.; Mecha, J.; Nirmalan, M.; Lu, J.R. A Technical Review of Face Mask Wearing in Preventing Respiratory COVID-19 Transmission. Curr. Opin. Colloid Interface Sci. 2021, 52, 101417. [Google Scholar] [CrossRef]
- Selvaranjan, K.; Navaratnam, S.; Rajeev, P.; Ravintherakumaran, N. Environmental Challenges Induced by Extensive Use of Face Masks during COVID-19: A Review and Potential Solutions. Environ. Chall. 2021, 3, 100039. [Google Scholar] [CrossRef]
- Xiong, S.W.; Fu, P.G.; Zou, Q.; Chen, L.Y.; Jiang, M.Y.; Zhang, P.; Wang, Z.G.; Cui, L.S.; Guo, H.; Gai, J.G. Heat Conduction and Antibacterial Hexagonal Boron Nitride/Polypropylene Nanocomposite Fibrous Membranes for Face Masks with Long-Time Wearing Performance. ACS Appl. Mater. Interfaces 2020, 13, 196–206. [Google Scholar] [CrossRef]
- Ray, S.S.; Park, Y.I.; Park, H.; Nam, S.E.; Kim, I.C.; Kwon, Y.N. Surface Innovation to Enhance Anti-Droplet and Hydrophobic Behavior of Breathable Compressed-Polyurethane Masks. Environ. Technol. Innov. 2020, 20, 101093. [Google Scholar] [CrossRef]
- Xiong, J.; Shao, W.; Wang, L.; Cui, C.; Jin, Y.; Yu, H.; Han, P.; Gao, Y.; Liu, F.; Ni, Q.; et al. PAN/FPU Composite Nanofiber Membrane with Superhydrophobic and Superoleophobic Surface as a Filter Element for High-Efficiency Protective Masks. Macromol. Mater. Eng. 2021, 306, 202100371. [Google Scholar] [CrossRef]
- Li, Y.; Yin, X.; Si, Y.; Yu, J.; Ding, B. All-Polymer Hybrid Electret Fibers for High-Efficiency and Low-Resistance Filter Media. Chem. Eng. J. 2020, 398, 125626. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Yin, X.; Yu, J.; Ding, B. Electret Polyvinylidene Fluoride Nanofibers Hybridized by Polytetrafluoroethylene Nanoparticles for High-Efficiency Air Filtration. ACS Appl. Mater. Interfaces 2016, 8, 23985–23994. [Google Scholar] [CrossRef]
- Han, K.S.; Lee, S.; Kim, M.; Park, P.; Lee, M.H.; Nah, J. Electrically Activated Ultrathin PVDF-TrFE Air Filter for High-Efficiency PM1.0 Filtration. Adv. Funct. Mater. 2019, 29, 1903633. [Google Scholar] [CrossRef]
- Karagoz, S.; Burak Kiremitler, N.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Serdar Onses, M.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, H.; Salam, A.; Hassan, T.; Munir, M.U.; Pasha, K.; Hassan, N. Fabrication of Promising Antimicrobial Aloe Vera/PVA Electrospun Nanofibers for Protective Clothing. Materials 2020, 13, 3884. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, T.J.; Ennis, S.; Musolino, S.F.; Buckley, H.L.; Niikura, M.; Wulff, J.E.; Menon, C. Covalent Functionalization of Polypropylene Filters with Diazirine–Photosensitizer Conjugates Producing Visible Light Driven Virus Inactivating Materials. Sci. Rep. 2021, 11, 19029. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, Z.; El-Moghazy, A.Y.; Wisuthiphaet, N.; Nitin, N.; Castillo, D.; Murphy, B.G. Daylight-Induced Antibacterial and Antiviral Nanofibrous Membranes Containing Vitamin K Derivatives for Personal Protective Equipment. ACS Appl. Mater. Interfaces 2020, 12, 49416–49430. [Google Scholar]
- Aragaw, T.A. Surgical Face Masks as a Potential Source for Microplastic Pollution in the COVID-19 Scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Seidi, F.; Yong, Q.; Jin, X.; Li, C.; Zheng, L.; Yuan, Z.; Xiao, H. Virucidal and Biodegradable Specialty Cellulose Nonwovens as Personal Protective Equipment against COVID-19 Pandemic. J. Adv. Res. 2021, 39, 147–156. [Google Scholar] [CrossRef]
- Gope, D.; Gope, A.; Gope, P.C. Mask Material: Challenges and Virucidal Properties as an Effective Solution against Coronavirus SARS-CoV-2. Open Health 2021, 1, 37–50. [Google Scholar] [CrossRef]
- Tiliket, G.; Le Sage, D.; Moules, V.; Rosa-Calatrava, M.; Lina, B.; Valleton, J.M.; Nguyen, Q.T.; Lebrun, L. A New Material for Airborne Virus Filtration. Chem. Eng. J. 2011, 173, 341–351. [Google Scholar] [CrossRef]
- Pullangott, G.; Kannan, U.; Gayathri, S.; Kiran, D.V.; Maliyekkal, S.M. A Comprehensive Review on Antimicrobial Face Masks: An Emerging Weapon in Fighting Pandemics. RSC Adv. 2021, 11, 6544–6576. [Google Scholar] [CrossRef]
- Catel-Ferreira, M.; Tnani, H.; Hellio, C.; Cosette, P.; Lebrun, L. Antiviral Effects of Polyphenols: Development of Bio-Based Cleaning Wipes and Filters. J. Virol. Methods 2015, 212, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral Effect of Catechins in Green Tea on Influenza Virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Bataglioli, R.A.; Rocha Neto, J.B.M.; Calais, G.B.; Lopes, L.M.; Tsukamoto, J.; de Moraes, A.P.; Arns, C.W.; Beppu, M.M. Hybrid Alginate–Copper Sulfate Textile Coating for Coronavirus Inactivation. J. Am. Ceram. Soc. 2022, 105, 1748–1752. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Rani, I.; Goyal, A.; Bhatnagar, M.; Manhas, S.; Goel, P.; Pal, A.; Prasad, R. Potential Molecular Mechanisms of Zinc- and Copper-Mediated Antiviral Activity on COVID-19. Nutr. Res. 2021, 92, 109–128. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Kumar, R.; Masand, R.; Rana, J.; Yatoo, M.I.; Tiwari, R.; Sharun, K.; Mohapatra, R.K.; Natesan, S.; et al. The Role of Disinfectants and Sanitizers during COVID-19 Pandemic: Advantages and Deleterious Effects on Humans and the Environment. Environ. Sci. Pollut. Res. 2021, 28, 34211–34228. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sher, F.; Zafar, A.; Lima, E.C. Environmental and Health Impacts of Spraying COVID-19 Disinfectants with Associated Challenges. Environ. Sci. Pollut. Res. 2021, 29, 85648–85657. [Google Scholar] [CrossRef]
- Vijayan P, P.; Chithra, P.G.; Abraham, P.; Susan, J.; Maria, H.J.; Sreedevi, T.; Thomas, S. Nanocoatings: Universal Antiviral Surface Solution against COVID-19. Prog. Org. Coat. 2021, 163, 106670. [Google Scholar] [CrossRef]
- Hamouda, T.; Ibrahim, H.M.; Kafafy, H.H.; Mashaly, H.M.; Mohamed, N.H.; Aly, N.M. Preparation of Cellulose-Based Wipes Treated with Antimicrobial and Antiviral Silver Nanoparticles as Novel Effective High-Performance Coronavirus Fighter. Int. J. Biol. Macromol. 2021, 181, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pi, Q.M.; You, H.H.; Li, J.Q.; Wang, P.C.; Yang, X.; Wu, Y. A Smart Multi-Functional Coating Based on Anti-Pathogen Micelles Tethered with Copper Nanoparticles: Via a Biosynthesis Method Using l-Vitamin C. RSC Adv. 2018, 8, 18272–18283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Comirnaty Comirnaty, INN-Tozinameran. EMA Eur. 2021. Available online: https://www.ema.europa.eu (accessed on 14 October 2022).
- Spikevax, INN-COVID-19 mRNA Vaccine (Nucleoside Modified). EMA Eur. 2021. Available online: https://www.ema.europa.eu (accessed on 14 October 2022).
- Katella, K. Comparing the COVID-19 Vaccines: How Are They Different? Yale Med. 2021, 22p. Available online: https://www.yalemedicine.org (accessed on 14 October 2022). [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S.; Firouzabadi, N.; Dehshahri, A.; Vazin, A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021, 100, 108162. [Google Scholar] [CrossRef]
- Anand, U.; Jakhmola, S.; Indari, O.; Jha, H.C.; Chen, Z.S.; Tripathi, V.; Pérez de la Lastra, J.M. Potential Therapeutic Targets and Vaccine Development for SARS-CoV-2/COVID-19 Pandemic Management: A Review on the Recent Update. Front. Immunol. 2021, 12, 658519. [Google Scholar] [CrossRef]
- Zahid, M.N.; Moosa, M.S.; Perna, S.; Buti, E.B. A Review on COVID-19 Vaccines: Stages of Clinical Trials, Mode of Actions and Efficacy. Arab J. Basic Appl. Sci. 2021, 28, 225–233. [Google Scholar] [CrossRef]
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakanthan, S. Review of COVID-19 Vaccine Subtypes, Efficacy and Geographical Distributions. Postgrad. Med. J. 2021, 98, 389–394. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Ng, S.W.; Singh, S.K.; Gulati, M.; Gupta, G.; Chaudhary, S.K.; Hing, G.B.; Collet, T.; MacLoughlin, R.; Lobenberg, R.; et al. Revolutionizing Polymer-Based Nanoparticle-Linked Vaccines for Targeting Respiratory Viruses: A Perspective. Life Sci. 2021, 280, 119744. [Google Scholar] [CrossRef] [PubMed]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A Review of the Antiviral Activity of Chitosan, Including Patented Applications and Its Potential Use against COVID-19. J. Appl. Microbiol. 2021, 132, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Jearanaiwitayakul, T.; Seesen, M.; Chawengkirttikul, R.; Limthongkul, J.; Apichirapokey, S.; Sapsutthipas, S.; Phumiamorn, S.; Sunintaboon, P.; Ubol, S. Intranasal Administration of Rbd Nanoparticles Confers Induction of Mucosal and Systemic Immunity against Sars-Cov-2. Vaccines 2021, 9, 768. [Google Scholar] [CrossRef]
- Dhama, K.; Dhawan, M.; Tiwari, R.; Emran, T.B.; Mitra, S.; Rabaan, A.A.; Alhumaid, S.; Al Alawi, Z.; Al Mutair, A. COVID-19 Intranasal Vaccines: Current Progress, Advantages, Prospects, and Challenges. Hum. Vaccin. Immunother. 2022, 18, 2045853. [Google Scholar] [CrossRef]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 Vaccines: From Bench to Bed. eBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, X.; Zhang, C.; Shao, X.; Zhang, X.; Zhang, Q. Conjugating Influenza A (H1N1) Antigen to N-Trimethylaminoethylmethacrylate Chitosan Nanoparticles Improves the Immunogenicity of the Antigen After Nasal Administration. J. Med. Virol. 2015, 87, 1807–1815. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Dehghan, S.; Kheiri, M.T.; Abnous, K.; Eskandari, M.; Tafaghodi, M. Preparation, Characterization and Immunological Evaluation of Alginate Nanoparticles Loaded with Whole Inactivated Influenza Virus: Dry Powder Formulation for Nasal Immunization in Rabbits. Microb. Pathog. 2018, 115, 74–85. [Google Scholar] [CrossRef]
- Boesteanu, A.C.; Babu, N.S.; Wheatley, M.; Papazoglou, E.S.; Katsikis, P.D. Biopolymer Encapsulated Live Influenza Virus as a Universal CD8+ T Cell Vaccine against Influenza Virus. Vaccine 2010, 29, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Moakes, R.J.A.; Davies, S.P.; Stamataki, Z.; Grover, L.M. Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-COV-2. Adv. Mater. 2021, 33, e202008304. [Google Scholar] [CrossRef] [PubMed]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; Panhuis, M.I.H. Enhanced Gelation Properties of Purified Gellan Gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan Gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A Review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, R.; Shrivastava, R.; Johansen, B.; Allain, T. Anti-Inflammatory and Antiviral Osmotic Polymeric Film to Treat Covid-19 Early-Stage Infection. J. Inflamm. Res. 2021, 14, 1195. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Hernández, L.M.; Ramírez-Noyola, J.A.; Gómez-García, I.A.; Ignacio-Cortés, S.; Zúñiga, J.; Choreño-Parra, J.A. Comparing the Cytokine Storms of COVID-19 and Pandemic Influenza. J. Interf. Cytokine Res. 2022, 42, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Bortolasci, C.C.; Puri, B.K.; Marx, W.; O’Neil, A.; Athan, E.; Walder, K.; Berk, M.; Olive, L.; Carvalho, A.F.; et al. The Cytokine Storms of COVID-19, H1N1 Influenza, CRS and MAS Compared. Can One Sized Treatment Fit All? Cytokine 2021, 144, 155593. [Google Scholar] [CrossRef]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Influenza Infection, SARS, MERS and COVID-19: Cytokine Storm—The Common Denominator and the Lessons to Be Learned. Clin. Immunol. 2021, 223, 108652. [Google Scholar] [CrossRef]
- Mudd, P.A.; Crawford, J.C.; Turner, J.S.; Souquette, A.; Reynolds, D.; Bender, D.; Bosanquet, J.P.; Anand, N.J.; Striker, D.A.; Martin, R.S.; et al. Distinct Inflammatory Profiles Distinguish COVID-19 from Influenza with Limited Contributions from Cytokine Storm. Sci. Adv. 2020, 6, abe3024. [Google Scholar] [CrossRef]
- Nandy Chatterjee, T.; Bandyopadhyay, R. A Molecularly Imprinted Polymer-Based Technology for Rapid Testing of COVID-19. Trans. Indian Natl. Acad. Eng. 2020, 5, 225–228. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Webster, T.J. The Promising Use of Nano-Molecular Imprinted Templates for Improved SARS-CoV-2 Detection, Drug Delivery and Research. J. Nanobiotechnol. 2021, 19, 305. [Google Scholar] [CrossRef] [PubMed]
- Wangchareansak, T.; Thitithanyanont, A.; Chuakheaw, D.; Gleeson, M.P.; Lieberzeit, P.A.; Sangma, C. A Novel Approach to Identify Molecular Binding to the Influenza Virus H5N1: Screening Using Molecularly Imprinted Polymers (MIPs). Medchemcomm 2014, 5, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Wangchareansak, T.; Thitithanyanont, A.; Chuakheaw, D.; Gleeson, M.P.; Lieberzeit, P.A.; Sangma, C. Influenza A Virus Molecularly Imprinted Polymers and Their Application in Virus Sub-Type Classification. J. Mater. Chem. B 2013, 1, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Ayankojo, A.G.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Molecularly Imprinted Polymer Based Electrochemical Sensor for Quantitative Detection of SARS-CoV-2 Spike Protein. Sens. Actuators B Chem. 2022, 353, 131160. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. Development of a Low-Cost Cotton-Tipped Electrochemical Immunosensor for the Detection of SARS-CoV-2. Anal. Chem. 2021, 93, 1826–1833. [Google Scholar] [CrossRef]
- Park, G.; Kim, H.O.; Lim, J.W.; Park, C.; Yeom, M.; Song, D.; Haam, S. Rapid Detection of Influenza A (H1N1) Virus by Conductive Polymer-Based Nanoparticle via Optical Response to Virus-Specific Binding. Nano Res. 2021, 15, 2254–2262. [Google Scholar] [CrossRef]
- Chen, P.L.; Lee, N.Y.; Cia, C.T.; Ko, W.C.; Hsueh, P.R. A Review of Treatment of Coronavirus Disease 2019 (COVID-19): Therapeutic Repurposing and Unmet Clinical Needs. Front. Pharmacol. 2020, 11, 584956. [Google Scholar] [CrossRef]
- Han, F.; Liu, Y.; Mo, M.; Chen, J.; Wang, C.; Yang, Y.; Wu, J. Current Treatment Strategies for COVID-19 (Review). Mol. Med. Rep. 2021, 24, 858. [Google Scholar] [CrossRef]
- Rodriguez-Guerra, M.; Jadhav, P.; Vittorio, T.J. Current Treatment in COVID-19 Disease: A Rapid Review. Drugs Context 2021, 10. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piatek, J. Hyaluronic Acid as a Component of Natural Polymer Blends for Biomedical Applications: A Review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef]
- Thirumalaisamy, R.; Aroulmoji, V.; Iqbal, M.N.; Deepa, M.; Sivasankar, C.; Khan, R.; Selvankumar, T. Molecular Insights of Hyaluronic Acid-Hydroxychloroquine Conjugate as a Promising Drug in Targeting SARS-CoV-2 Viral Proteins. J. Mol. Struct. 2021, 1238, 130457. [Google Scholar] [CrossRef]
- Surnar, B.; Kamran, M.Z.; Shah, A.S.; Dhar, S. Clinically Approved Antiviral Drug in an Orally Administrable Nanoparticle for COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 1371–1380. [Google Scholar] [CrossRef]
- Heidary, F.; Gharebaghi, R. Ivermectin: A Systematic Review from Antiviral Effects to COVID-19 Complementary Regimen. J. Antibiot. 2020, 73, 593–602. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Zhang, Q.; Honko, A.; Zhou, J.; Gong, H.; Downs, S.N.; Vasquez, J.H.; Fang, R.H.; Gao, W.; Griffiths, A.; Zhang, L. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett. 2020, 20, 5570–5574. [Google Scholar] [CrossRef]
- Park, J.H.; Mohapatra, A.; Zhou, J.; Holay, M.; Krishnan, N.; Gao, W.; Fang, R.H.; Zhang, L. Virus-Mimicking Cell Membrane-Coated Nanoparticles for Cytosolic Delivery of MRNA. Angew. Chem. Int. Ed. 2022, 134, e202113671. [Google Scholar] [CrossRef]




| Strategies | Virus | Type of Polymer | Results | Ref. |
|---|---|---|---|---|
| Face masks | SARS-CoV-2 | Synthetic (polypropylene, polyurethane, polyacrylonitrile, polystyrene) | Effective in viral inactivation but associated with environmental pollution (affecting both human and animal health). | [40,41,42,43] |
| Natural (cellulose, alginate) | The BCNWs inactivated >99% of the viruses, while the fabrics coated with alginate–Cu(II) presented a 99% viral inactivation when in contact with the virus for less than 1 min. | [51] | ||
| Influenza A | Natural (cellulose) | The filters proved to not only to block the droplet-borne/airborne IAV, but also to remove 99.999% of a sprayed solution of T4D bacteriophages after 1 h. | [53] | |
| L. innocua * | Synthetic (polyacrylonitrile and poly(vinylalcohol-co-ethylene)) | The nanofibers membranes showed a high antiviral efficiency of >99.9% within a short exposure time (<90 min). | [49] | |
| Protective clothing | SARS-CoV-2 Influenza A | Synthetic (poly(methyl methacrylate)) | The PMMA nanofibers showed high performance as an antiviral agent. | [46] |
| SARS-CoV-2 | Synthetic (polyvinyl alcohol) | With a 3% concentration of AV, the antibacterial activity of the nanofibers was excellent, with high zone of inhibition values of 10.50 (trial 1), 10.79 (trial 2) and 11.08 mm (trial 3). | [47] | |
| Disinfectants | MERS-CoV | Natural (cellulose) | Anti-viral inhibitory effect of 48.3%. | [65] |
| Influenza A | Synthetic (polyoxyethylene–polyoxypropylene) | In a short period of time (1 min), IAV was killed. | [66] | |
| Vaccines | SARS-CoV-2 | Synthetic (polyethylene-glycol) | Effective, but associated with allergic reactions. | [68,69] |
| Natural or derivative(chitosan) | On day 45 after intranasal immunization, the mucosal, systemic humoral and cell-mediated immune responses were highly stimulated (with a robust production of immunoglobulins or CD8+ cells). | [78] | ||
| Influenza A | Natural or derivative (N,N,N-trimethyl chitosan) | Optimal immune response with the production of IL-1β (by macrophages) at 16 mg/mL and IL-2 (by lymphocytes) at 64 mg/mL. | [81] | |
| Natural (alginate) | On day 7 post challenge, the frequency of lung CD8+ cells was 26 ± 2.7% in mice that received the polymer encapsulated vaccine, comparing to mice injected with alginate alone (1.4 ± 0.07%) or non-vaccinated mice (2 ± 0.6%). | [84] | ||
| Nasal spray | SARS-CoV-2 | Natural (gellan gum and carrageenan) | Potent antiviral spray (in a proportion of 75:25, gellan to λ-carrageenan) with protective and inhibitory effects against SARS-CoV-2 (suppression of the infection up to a dilution of 1/100 in comparison with the untreated control group). | [85] |
| Natural (hydroxypropyl cellulose and solagum) | At 5% concentration, the final Covispray composition neutralized nearly 30% of IL-6, 80% of TNF-α and GM-CSF, as well as more than 90% of pro-inflammatory cytokines. | [89] |
| Strategies | Virus | Type of Polymer | Results | Ref. |
|---|---|---|---|---|
| MIPs | SARS-CoV-2 | Synthetic (poly-3-aminophenylboronic acid) | The biosensor demonstrated a rebinding time of 15 min and a measurement duration of 5 min, being comparable with the current available antigen testing assays. | [98] |
| Influenza A | Synthetic (polyacrylamide, poly-methacrylic acid, poly-methylmethacrylate and poly-N-vinylpyrrolidone) | Each MIP possessed a better recognition property towards its original viral template. A fully horizontal response was obtained after 3–4 h. | [97] | |
| Electrochemical immunosensor | SARS-CoV-2 | Natural (cotton fibers) | The biosensor showed a very good sensitivity, with a LOD of 0.8 pg/mL, and also a high selectivity, since it did not show cross-reactivity with antigens from other tested viruses. | [99] |
| Diagnosis platform | Influenza A | Synthetic (poly(aniline-co-pyrrole)) | The detection system possesses a sufficient level of LOD (3.37 log10 TCID50/mL) and a target-responsive signal transduction. | [100] |
| Strategies | Virus | Type of Polymer | Results | Study Type | Ref. |
|---|---|---|---|---|---|
| Drug-polymer conjugate | SARS-CoV-2 | Natural (Hyaluronic acid) | Superior binding affinity of the conjugates (ranging from −13.2046 KJ/mol to −23.1778 KJ/mol), comparing to free HCQ drug (ranging from −12.2217 KJ/mol to −13.6327 KJ/mol). | In silico | [109] |
| Loaded NPs | SARS-CoV-2 | Synthetic (PLGA-PEG-Mal) | At 4 h treatment, the IVM-NP was able to decrease the expression of viral spike protein and its receptor angiotensin-converting enzyme 2. | In vitro In vivo | [110] |
| Influenza A | Natural (Alginate) | Strong humoral and cellular immune response with the activation of B and dendritic cells, as well as secretion of cytokines. | In vitro In vivo | [83] | |
| Cellular nanosponges | SARS-CoV-2 | Synthetic (PLGA) | The cell membranes showed comparable ability to neutralize the virus with IC50 values of 827.1 µg/mL and 882.7 µg/mL for E-NS and MΦ-NS, respectively. Neutralization of the virus occurs in a concentration-dependent manner. | In vitro In vivo | [113] |
| Influenza A | Synthetic (PLGA) | The in vitro activity resulted on a successful expression of hemagglutinin. The in vivo activity showed a significant increase in protein expression (increase in bioluminescence at 24 h) in both local and systemic delivery scenarios. | In vitro In vivo | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.C.F.; Martel, F.; Freire, C.S.R.; Ferreira, B.J.M.L. Polymeric Materials as Indispensable Tools to Fight RNA Viruses: SARS-CoV-2 and Influenza A. Bioengineering 2022, 9, 816. https://doi.org/10.3390/bioengineering9120816
Santos ACF, Martel F, Freire CSR, Ferreira BJML. Polymeric Materials as Indispensable Tools to Fight RNA Viruses: SARS-CoV-2 and Influenza A. Bioengineering. 2022; 9(12):816. https://doi.org/10.3390/bioengineering9120816
Chicago/Turabian StyleSantos, Ariana C. F., Fátima Martel, Carmen S. R. Freire, and Bárbara J. M. L. Ferreira. 2022. "Polymeric Materials as Indispensable Tools to Fight RNA Viruses: SARS-CoV-2 and Influenza A" Bioengineering 9, no. 12: 816. https://doi.org/10.3390/bioengineering9120816
APA StyleSantos, A. C. F., Martel, F., Freire, C. S. R., & Ferreira, B. J. M. L. (2022). Polymeric Materials as Indispensable Tools to Fight RNA Viruses: SARS-CoV-2 and Influenza A. Bioengineering, 9(12), 816. https://doi.org/10.3390/bioengineering9120816