Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study
Abstract
:1. Introduction
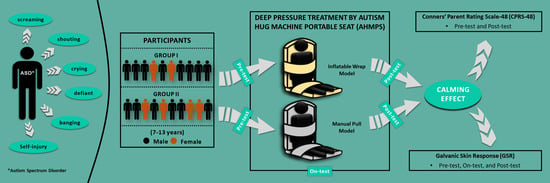
2. Materials and Methods
2.1. Study Design and Population
2.2. Instruments
2.2.1. Autism Hug Machine Portable Seat (AHMPS)
2.2.2. Conners’ Parent Rating Scale-48 (CPRS-48)
2.2.3. Galvanic Skin Response (GSR)
2.3. Procedures
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Baio, J. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. Morb. Mortal. Wkly. Rep. 2014, 63, 1–21. [Google Scholar]
- Muhle, R.; Trentacoste, S.V.; Rapin, I. The Genetics of Autism. Pediatrics 2004, 113, e472-86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Stavinoha, P.L.; Askins, M.A.; Powell, S.K.; Smiley, N.P.; Robert, R.S. Neurocognitive and Psychosocial Outcomes in Pediatric Brain Tumor Survivors. Bioengineering 2018, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Duffney, L.J.; Valdez, P.; Tremblay, M.W.; Cao, X.; Montgomery, S.; McConkie-Rosell, A.; Jiang, Y. Epigenetics and Autism Spectrum Disorder: A Report of an Autism Case with Mutation in H1 Linker Histone HIST1H1E and Literature Review. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Van de Water, J. Maternal Autoantibody Related Autism: Mechanisms and Pathways. Mol. Psychiatry 2019, 24, 252–265. [Google Scholar] [CrossRef]
- Jones, H.F.; Ho, A.C.C.; Sharma, S.; Mohammad, S.S.; Kothur, K.; Patel, S.; Brilot, F.; Guastella, A.J.; Dale, R.C. Maternal Thyroid Autoimmunity Associated with Acute-Onset Neuropsychiatric Disorders and Global Regression in Offspring. Dev. Med. Child Neurol. 2019, 61, 984–988. [Google Scholar] [CrossRef]
- Vogel Ciernia, A.; Careaga, M.; LaSalle, J.M.; Ashwood, P. Microglia from Offspring of Dams with Allergic Asthma Exhibit Epigenomic Alterations in Genes Dysregulated in Autism. Glia 2018, 66, 505–521. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in Autism Spectrum Disorder-a Translational Magnetic Resonance Spectroscopy Study in Man and Rodent Models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Rojas, D.C.; Singel, D.; Steinmetz, S.; Hepburn, S.; Brown, M.S. Decreased Left Perisylvian GABA Concentration in Children with Autism and Unaffected Siblings. NeuroImage 2014, 86, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the Sensory Abnormalities of Children and Adults with Autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Kojovic, N.; Ben Hadid, L.; Franchini, M.; Schaer, M. Sensory Processing Issues and Their Association with Social Difficulties in Children with Autism Spectrum Disorders. J. Clin. Med. 2019, 8, 1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soler, N.; Hardwick, C.; Perkes, I.E.; Mohammad, S.S.; Dossetor, D.; Nunn, K.; Bray, P.; Dale, R.C. Sensory Dysregulation in Tic Disorders Is Associated with Executive Dysfunction and Comorbidities. Mov. Disord. 2019, 34, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-W.; Rodger, S.; Copley, J.; Branjerdporn, G.; Taggart, C. Sensory Processing and Its Relationship with Children’s Daily Life Participation. Phys. Occup. Ther. Pediatr. 2016, 36, 73–87. [Google Scholar] [CrossRef]
- Yonkman, J.; Lawler, B.; Talty, J.; O’Neil, J.; Bull, M. Safely Transporting Children with Autism Spectrum Disorder: Evaluation and Intervention. Am. J. Occup. Ther. 2013, 67, 711–716. [Google Scholar] [CrossRef] [Green Version]
- Peña, M.; Ng, Y.; Ripat, J.; Anagnostou, E. Brief Report: Parent Perspectives on Sensory-Based Interventions for Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 8, 106. [Google Scholar] [CrossRef]
- Ayres, A.J. Sensory Integration and Learning Disorders; Western Psychological Services: Vancouver, WA, USA, 1972. [Google Scholar]
- Grandin, T. Calming Effects of Deep Touch Pressure in Patients with Autistic Disorder, College Students, and Animals. J. Child Adolesc. Psychopharmacol. 1992, 2, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Schaffler, M.D.; Middleton, L.J.; Abdus-Saboor, I. Mechanisms of Tactile Sensory Phenotypes in Autism: Current Understanding and Future Directions for Research. Curr. Psychiatry Rep. 2019, 21, 134. [Google Scholar] [CrossRef] [Green Version]
- Cortelli, P.; Giannini, G.; Favoni, V.; Cevoli, S.; Pierangeli, G. Nociception and Autonomic Nervous System. Neurol. Sci. 2013, 34, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, T.; Hernandez-Reif, M.; Diego, M.; Schanberg, S.; Kuhn, C. Cortisol Decreases and Serotonin and Dopamine Increase Following Massage Therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Spriggs, A.D.; Ault, M.J.; Flanagan, S.; Sartini, E.C. A Systematic Review of Weighted Vests with Individuals with Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2017, 37, 49–60. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of Bottom Profile Dimples on the Femoral Head on Wear in Metal-on-Metal Total Hip Arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Basri, H.; Syahrom, A.; Prakoso, A.T.; Wicaksono, D.; Amarullah, M.I.; Ramadhoni, T.S.; Nugraha, R.D. The Analysis of Dimple Geometry on Artificial Hip Joint to the Performance of Lubrication. J. Phys. Conf. Ser. 2019, 1198, 1–10. [Google Scholar] [CrossRef]
- Basri, H.; Syahrom, A.; Ramadhoni, T.S.; Prakoso, A.T.; Ammarullah, M.I.; Vincent. The Analysis of the Dimple Arrangement of the Artificial Hip Joint to the Performance of Lubrication. IOP Conf. Ser. Mater. Sci. Eng. 2019, 620, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Basri, H. Contact Pressure Analysis of Acetabular Cup Surface with Dimple Addition on Total Hip Arthroplasty Using Finite Element Method. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1034, 1–11. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Bayuseno, A.P.; Basri, H.; Syahrom, A.; Saad, A.P.D.; Jamari, J. 2D Computational Tresca Stress Prediction of CoCrMo-on- UHMWPE Bearing of Total Hip Prosthesis Based on Body Mass Index. Malaysian J. Med. Health Sci. 2021, 17 (Suppl. 13), 18–21. [Google Scholar]
- Akbar, I.; Prakoso, A.T.; Astrada, Y.M.; Sinaga, M.S.; Ammarullah, M.I.; Adanta, D.; Mataram, A.; Syahrom, A.; Jamari, J.; Basri, H. Permeability Study of Functionally Graded Scaffold Based on Morphology of Cancellous Bone. Malaysian J. Med. Health Sci. 2021, 17 (Suppl. 13), 60–66. [Google Scholar]
- Jamari, J.; Ammarullah, M.I.; Afif, I.Y.; Ismail, R.; Tauviqirrahman, M.; Bayuseno, A.P. Running-in Analysis of Transmission Gear. Tribol. Ind. 2021, 43, 434–441. [Google Scholar] [CrossRef]
- Wang, C.-A.; Baird, T.; Huang, J.; Coutinho, J.D.; Brien, D.C.; Munoz, D.P. Arousal Effects on Pupil Size, Heart Rate, and Skin Conductance in an Emotional Face Task. Front. Neurol. 2018, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, K.; Resch, B.; Sagl, G.; Petutschnig, A.; Werner, C.; Niederseer, D.; Liedlgruber, M.; Wilhelm, F.H.; Osborne, T.; Pykett, J. Detecting Moments of Stress from Measurements of Wearable Physiological Sensors. Sensors 2019, 19, 3805. [Google Scholar] [CrossRef] [Green Version]
- Afif, I.Y.; Maula, M.I.; Aliyafi, M.B.; Aji, A.L.; Winarni, T.I.; Jamari, J. Design of Hug Machine Portable Seat for Autistic Children in Public Transport Application. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1096, p. 12034. [Google Scholar]
- Maula, M.I.; Aji, A.L.; Aliyafi, M.B.; Afif, I.Y.; Ammarullah, M.I.; Winarni, T.I.; Jamari, J. The Subjective Comfort Test of Autism Hug Machine Portable Seat. J. Intellect. Disabil.-Diagnosis Treat. 2021, 9, 182–188. [Google Scholar] [CrossRef]
- Foo, E.; Lee, J.W.; Ozbek, S.; Holschuh, B. Preliminary Study of the Subjective Comfort and Emotional Effects of On-Body Compression. In Proceedings of the 2018 ACM International Symposium on Wearable Computers, Heidelberg, Germany, 8–12 October 2018; pp. 128–131. [Google Scholar]
- Goyette, C.H.; Conners, C.K.; Ulrich, R.F. Normative Data on Revised Conners Parent and Teacher Rating Scales. J. Abnorm. Child Psychol. 1978, 6, 221–236. [Google Scholar] [CrossRef]
- Edelson, S.M.; Edelson, M.G.; Kerr, D.C.R.; Grandin, T. Behavioral and Physiological Effects of Deep Pressure on Children with Autism: A Pilot Study Evaluating the Efficacy of Grandin’s Hug Machine. Am. J. Occup. Ther. 1999, 53, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; SAGE: London, UK, 2013. [Google Scholar]
- Hartley, S.L.; Schaidle, E.M.; Burnson, C.F. Parental Attributions for the Behavior Problems of Children and Adolescents with Autism Spectrum Disorders. J. Dev. Behav. Pediatr. 2013, 34, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, S.; Lane, S.J.; Mullen, B. Effects of Deep Pressure Stimulation on Physiological Arousal. Am. J. Occup. Ther. 2015, 69, 6903350010p1–6903350010p5. [Google Scholar] [CrossRef] [Green Version]
- Krauss, K.E. The Effects of Deep Pressure Touch on Anxiety. Am. J. Occup. Ther. 1987, 41, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, G.; Lamm, C.; Brodbeck, C.; Singer, T. Skin Conductance Response to the Pain of Others Predicts Later Costly Helping. PLoS ONE 2011, 6, e22759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, A.A.; Ghobashy, M.A.M.; Ouda, W.; Stout, R.G.; Silverman, D.G.; Shelley, K.H. Different Responses of Ear and Finger Pulse Oximeter Wave Form to Cold Pressor Test. Anesth. Analg. 2001, 92, 1483–1486. [Google Scholar] [CrossRef]
- Minoura, M.; Tani, I.; Ishii, T.; Gunji, Y.-P. Observing the Transformation of Bodily Self-Consciousness in the Squeeze-Machine Experiment. J. Vis. Exp. 2019, 145, e59263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, L.J. Facilitating Neurodevelopment. Proc. Autism Soc. Am. 1989, 1, 117–120. [Google Scholar]



| Subscales | Group | N | Pretest (Mean ± SD) | Posttest (Mean ± SD) | Mean Difference | p |
|---|---|---|---|---|---|---|
| Conduct problems | I | 10 | 1.441 ± 0.442 | 1.176 ± 0.158 | −0.482 | 0.007 * |
| II | 10 | 1.866 ± 0.498 | 1.640 ± 0.435 | |||
| Learning problems | I | 10 | 2.250 ± 0.333 | 2.075 ± 0.442 | −0.400 | 0.060 |
| II | 10 | 2.725 ± 0.629 | 2.400 ± 0.459 | |||
| Psychosomatic problems | I | 10 | 1.475 ± 0.546 | 1.225 ± 0.362 | −0.001 | 0.990 |
| II | 10 | 1.400 ± 0.394 | 1.300 ± 0.405 | |||
| Impulsive–hyperactivity behaviors | I | 10 | 2.450 ± 0.575 | 2.025 ± 0.381 | −0.162 | 0.500 |
| II | 10 | 2.575 ± 0.708 | 2.225 ± 0.583 | |||
| Anxiety | I | 10 | 2.050 ± 0.497 | 1.700 ± 0.422 | −0.325 | 0.250 |
| II | 10 | 2.350 ±0.851 | 2.050 ± 0.725 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afif, I.Y.; Farkhan, M.; Kurdi, O.; Maula, M.I.; Ammarullah, M.I.; Setiyana, B.; Jamari, J.; Winarni, T.I. Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering 2022, 9, 48. https://doi.org/10.3390/bioengineering9020048
Afif IY, Farkhan M, Kurdi O, Maula MI, Ammarullah MI, Setiyana B, Jamari J, Winarni TI. Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering. 2022; 9(2):48. https://doi.org/10.3390/bioengineering9020048
Chicago/Turabian StyleAfif, Ilham Yustar, Muhammad Farkhan, Ojo Kurdi, Mohamad Izzur Maula, Muhammad Imam Ammarullah, Budi Setiyana, J. Jamari, and Tri Indah Winarni. 2022. "Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study" Bioengineering 9, no. 2: 48. https://doi.org/10.3390/bioengineering9020048
APA StyleAfif, I. Y., Farkhan, M., Kurdi, O., Maula, M. I., Ammarullah, M. I., Setiyana, B., Jamari, J., & Winarni, T. I. (2022). Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering, 9(2), 48. https://doi.org/10.3390/bioengineering9020048