Biosensor-Based Approaches for Detecting Ochratoxin A and 2,4,6-Trichloroanisole in Beverages
Abstract
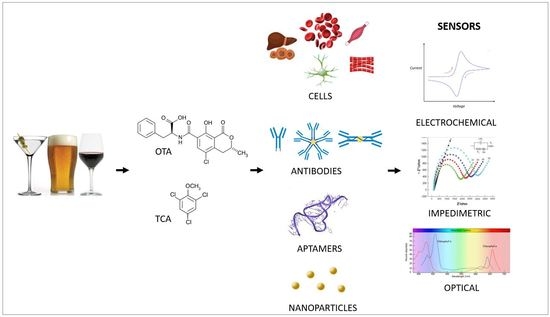
:1. Introduction
2. Biosensors for the Detection of Ochratoxin A in Wine, Beer and Other Beverages
3. Biosensors for the Detection of Haloanisoles in Wine and Beverages
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bennet, J.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Venâncio, A.; Paterson, R. The challenge of mycotoxins. In Food Safety—A Practical and Case Study Approach; McElhatton, A., Marshall, R., Eds.; Springer: Heidelberg, Germany, 2007; Volume 1, pp. 24–47. ISBN 978-0-387-33957-3. [Google Scholar]
- Bhat, R.; Rai, R.; Karim, A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Aflatoxins (sum of B1, B2, G1, G2) in cereals and cereal-derived food products. EFSA Support. Publ. 2013, 406, 1–11. [Google Scholar] [CrossRef]
- Milićević, D.; Škrinjar, M.; Baltić, T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins 2010, 2, 572–592. [Google Scholar] [CrossRef] [PubMed]
- Richard, J. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA J. 2014, 12, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Cravero, M.C.; Bonello, F.; Pazo Alvarez del Carmen, M.; Tsolakis, C.; Borsa, D. The sensory evaluation of 2,4,6-trichloroanisole in wines. J. Inst. Brew. 2015, 121, 411–417. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Baumgartner, S.; Josephs, R. The state-of-the-art in the analysis of type-A and -B trichothecene mycotoxins in cereals. Fresenius J. Anal. Chem. 2001, 371, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Krska, R.; Sulyok, M. Mycotoxin Contamination in Sugarcane Grass and Juice: First Report on Detection of Multiple Mycotoxins and Exposure Assessment for Aflatoxins B1 and G1 in Humans. Toxins 2016, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Arduini, F.; Palleschi, G.; Rea, G. Bio sensing technology for sustainable food safety. Trends Anal. Chem. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Thevenot, D.; Toth, K.; Durst, R.; Wilson, G. Electrochemical biosensors: Recommended definitions and classification. Anal. Lett. 2001, 34, 635–659. [Google Scholar] [CrossRef] [Green Version]
- Omrani, N.M.; Hayat, A.; Korri-Youssoufi, H.; Marty, J.L. Electrochemical biosensors for food security: Mycotoxins detection. In Biosensors for Security and Bioterrorism Applications; Nikolelis, D., Nikoleli, G.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 469–490. ISBN 978-3-319-28926-7. [Google Scholar]
- Bazin, I.; Tria, S.; Hayat, A.; Marty, J. New biorecognition molecules in biosensors for the detection of toxins. Biosens. Bioelectron. 2017, 87, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chang, H.; Lee, N.; Chun, H. A Surface Plasmon Resonance Sensor for the Detection of Deoxynivalenol Using a Molecularly Imprinted Polymer. Sensors 2011, 11, 8654–8664. [Google Scholar] [CrossRef] [PubMed]
- Buerk, D. Biosensors: Theory and Applications; CRC Press: Lancaster, PA, USA, 1995; pp. 6–15. ISBN 9780877629757. [Google Scholar]
- Koyun, A.; Ahlatcıoğlu, E.; İpek, Y. Biosensors and Their Principles. In A Roadmap of Biomedical Engineers and Milestones; Kara, S., Ed.; InTech: Vienna, Austria, 2012; ISBN 978-953-51-0609-8. [Google Scholar]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J. Determination of Mycotoxins in Food: A Review of Bioanalytical to Analytical Methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Ochratoxin A. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occurring Substances, Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; World Health Organization: Geneva, Switzerland, 1993; Volume 56, p. 489. ISBN 9789283212560. [Google Scholar]
- Drunday, V.; Pacin, A. Occurrence of Ochratoxin A in coffee beans, ground roasted coffee and soluble coffee and method validation. Food Control 2013, 30, 675–678. [Google Scholar] [CrossRef]
- Batista, L.R.; Chalfoun, S.M.; Silva, C.F.; Cirillo, M.; Varga, E.A.; Schwan, R.F. Ochratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control 2009, 20, 784–790. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T.; Hilts, C.; Billiard, S.; Kiparissis, Y.; Richard, I.; Hayward, S. Health risk assessment of ochratoxin A for all age-sex strata in a market economy. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess 2010, 27, 212–240. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; van Dam, R.; van Doorn, R.; Katerere, D.; Berthiller, F.; Nielen, M. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS ONE 2017, 12, e0185887. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nagatomi, Y.; Uyama, A.; Mochizuki, N. Fate of mycotoxins during beer brewing and fermentation. Biosci. Biotechnol. Biochem. 2013, 77, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Anli, R.; Vural, N.; Bayram, M. Removal of Ochratoxin A (OTA) from Naturally Contaminated Wines During the Vinification Process. J. Inst. Brew. 2011, 117, 456–461. [Google Scholar] [CrossRef]
- Dachery, B.; Manfroi, V.; Berleze, K.; Welke, J. Occurrence of ochratoxin A in grapes, juices and wines and risk assessment related to this mycotoxin exposure. Cienc. Rural 2016, 46, 176–183. [Google Scholar] [CrossRef]
- Bolton, S.; Mitchell, T.; Brannen, P.; Glenn, A. Assessment of Mycotoxins in Vitis vinifera Wines. Am. J. Enol. Vitic. 2017, 68, 336–343. [Google Scholar] [CrossRef]
- Schothorst, R.; van Egmond, H. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”. Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Safety evaluation of certain mycotoxins in food. In WHO Food Additives Series 63 and FAO Food and Nutrition; Prepared by the Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Rome, Italy, 2011; pp. 8, 353–365. ISBN 978-92-4-166063-1. [Google Scholar]
- Da Rocha, B.; da Chagas, F.; Freire, O.; Feitosa Maia, F.E.; Florindo Guedes, M.I.; Rondina, D.; Edite, M. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Prieto-Simón, B.; Campàs, M.; Marty, J.; Noguer, T. Novel highly-performing immunosensor-based strategy for ochratoxin A detection in wine samples. Biosens. Bioelectron. 2008, 23, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, S.; Micheli, L.; Palleschi, G.; Compagnone, D. Development of an Electrochemical Immunosensor for Ochratoxin A. Anal. Lett. 2004, 37, 1545–1558. [Google Scholar] [CrossRef]
- Vidal, J.; Bonel, L.; Ezquerra, A.; Duato, P.; Castillo, J. An electrochemical immunosensor for ochratoxin A determination in wines based on a monoclonal antibody and paramagnetic microbeads. Anal. Bioanal. Chem. 2012, 403, 1585. [Google Scholar] [CrossRef] [PubMed]
- Novo, P.; Moulas, G.; França Prazeres, D.; Chu, V.; Conde, J. Detection of ochratoxin A in wine and beer by chemiluminescence-based ELISA in microfluidics with integrated photodiodes. Sens. Actuators B Chem. 2013, 176, 232–240. [Google Scholar] [CrossRef]
- Pagkali, V.; Petrou, P.; Salapatas, A.; Makarona, E.; Peters, J.; Haasnoot, W.; Jobst, G.; Economou, A.; Misiakos, K.; Raptis, I.; et al. Detection of ochratoxin A in beer samples with a label-free monolithically integrated optoelectronic biosensor. J. Hazard. Mater. 2017, 323, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zong, Y.; Richter, R.; Knoll, W. Enzyme immobilization on poly(ethylene-co-acrylic acid) films studied by quartz crystal microbalance with dissipation monitoring. J. Colloid Interface Sci. 2005, 287, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Haupt, K.; Feller, K.H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Tang, T.; Harrison, D.; Lee, W.; Jemere, A.B. A regenerating ultrasensitive electrochemical impedance immunosensor for the detection of adenovirus. Biosens. Bioelectron. 2015, 68, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jarocka, U.; Sawicka, R.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecki, J.; Radecka, H. Electrochemical immunosensor for detection of antibodies against influenza A virus H5N1 in hen serum. Biosens. Bioelectron. 2014, 55, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Huang, Y.; Zhang, C.; Liu, H.; Tang, D. Amplified impedimetric immunosensor based on instant catalyst for sensitive determination of ochratoxin A. Biosens. Bioelectron. 2016, 86, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Chen, J.; Li, C.; Xie, G.; Fu, H.; Ma, Z.; Lu, A. Highly Sensitive Colorimetric Detection of Ochratoxin A by a Label-Free Aptamer and Gold Nanoparticles. Toxins 2015, 7, 5377–5385. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, S.; Liu, X.; He, C.; Tang, Y.; Li, Q.; Liu, J.; Su, H.; Tan, T.; Dong, Y. Aptamer-based Colorimetric Biosensing of Ochratoxin A in Fortified White Grape Wine Sample Using Unmodified Gold Nanoparticles. Anal. Sci. 2017, 33, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Long, F.; Zhou, X.; Shi, H. Portable detection of Ochratoxin A in red wine based on a structure-switching aptamer using a personal glucometer. RSC Adv. 2016, 6, 29563–29569. [Google Scholar] [CrossRef]
- Castillo, G.; Lamberti, I.; Mosiello, L.; Hianik, T. Impedimetric DNA Aptasensor for Sensitive Detection of Ochratoxin A in Food. Electroanalysis 2012, 24, 512–520. [Google Scholar] [CrossRef]
- Bueno, D.; Valdez, L.; Gutiérrez Salgado, J.M.; Marty, J.; Muñoz, R. Colorimetric Analysis of Ochratoxin A in Beverage Samples. Sensors 2016, 16, 1888. [Google Scholar] [CrossRef] [PubMed]
- Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th ed.; Budavari, S., O’Neil, M., Smith, A., Heckelman, P., Kinneary, J., Eds.; Merck Research Laboratories Division of Merck & Co.: Whitehouse Station, NJ, USA, 1996; ISBN 978-0911910124. [Google Scholar]
- Bueno, D.; Muñoz, R.; Marty, J. Fluorescence analyzer based on smartphone camera and wireless for detection of Ochratoxin A. Sens. Actuators B Chem. 2016, 232, 462–468. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, T.; Qu, Z.; Li, H.; Yang, Z. Contribution of filamentous fungi to the musty odorant 2,4,6-trichloroanisole in water supply reservoirs and associated drinking water treatment plants. Chemosphere 2017, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.; Peña-Neira, B.; Fernández de Simón, M.; García-Vallejo, T.; Hernández, E.; Cadahía, J. Presence of cork-taint responsible compounds in wines and their cork stoppers. Eur. Food Res. Technol. 2000, 211, 257–261. [Google Scholar] [CrossRef]
- Chatonnet, P.; Bonnet, S.; Boutou, S.; Labadie, M. Identification and responsibility of 2,4,6-tribromoanisole in musty, corked odors in wine. J. Agric. Food Chem. 2004, 52, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Sefton, M.; Simpson, R. Compounds causing cork taint and the factors affecting their transfer from natural cork closures to wine—A review. Aust. J. Grape Wine Res. 2005, 11, 226–240. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kato, H.; Kurahashi, T. 2,4,6-trichloroanisole is a potent suppressor of olfactory signal transduction. Proc. Natl. Acad. Sci. USA 2013, 110, 16235–16240. [Google Scholar] [CrossRef] [PubMed]
- Juanola, R.; Guerrero, L.; Subirà, D.; Salvadó, V.; Insa, S.; Garcia Regueiro, J.; Anticó, E. Relationship between sensory and instrumental analysis of 2,4,6-trichloroanisole in wine and cork stoppers. Anal. Chim. Acta 2004, 513, 291–297. [Google Scholar] [CrossRef]
- Fontana, A. Analytical methods for determination of cork-taint compounds in wine. Trends Anal. Chem. 2012, 37, 135–147. [Google Scholar] [CrossRef]
- Slabizki, P.; Fischer, C.; Legrum, C.; Schmarr, H. Characterization of Atypical Off-Flavor Compounds in Natural Cork Stoppers by Multidimensional Gas Chromatographic Techniques. J. Agric. Food. Chem. 2015, 63, 7840–7848. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Pérez-del-Notario, N.; González-Sáiz, J. Optimisation of a microwave-assisted extraction method for the simultaneous determination of haloanisoles and halophenols in cork stoppers. J. Chromatogr. A 2007, 1149, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Cork Stoppers—Determination of Releasable 2,4,6-Trichloroanisol (TCA), 2014; ISO20752:2014(E); International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Anonymous. Determination of Releasable 2,4,6-Trichloroanisole in Wine by Cork Stoppers; Method OIV-MA-AS315-16; Compendium of International Analysis of Methods: Paris, France, 2009. [Google Scholar]
- Vlachos, P.; Kampioti, A.; Kornaros, M.; Lyberatos, G. Matrix effect during the application of a rapid method using HS-SPME followed by GC–ECD for the analysis of 2,4,6-TCA in wine and cork soaks. Food Chem. 2007, 105, 681–690. [Google Scholar] [CrossRef]
- Jeleń, H.; Dziadas, M.; Majcher, M. Different headspace solid phase microextraction–gas chromatography/mass spectrometry approaches to haloanisoles analysis in wine. J. Chromatogr. A 2013, 1313, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Young, T.; Butzke, C.E. Supercritical fluid extraction of 2,4,6-trichloroanisole from cork stoppers. J. Agric. Food Chem. 2000, 48, 865–871. [Google Scholar] [CrossRef]
- Cacho, J.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Cloud point extraction and gas chromatography with direct microvial insert thermal desorption for the determination of haloanisoles in alcoholic beverages. Talanta 2016, 160, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ariza, J.; García-Barrera, T.; Lorenzo, F.; Beltrán, R. Use of multiple headspace solid-phase microextraction and pervaporation for the determination of off-flavours in wine. J. Chromatogr. A 2006, 1112, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Rauhut, D.; Jung, R. “Cork taint” responsible compounds. Determination of haloanisoles and halophenols in cork matrix: A review. Talanta 2017, 175, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Sánchez-Baeza, F.; Marco, M. Immunochemical determination of 2,4,6-trichloroanisole as the responsible agent for the musty odor in foods. 1. Molecular modeling studies for antibody production. J. Agric. Food Chem. 2003, 51, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Varela, B.; Marco, M. Immunochemical determination of 2,4,6-trichloroanisole as the responsible agent for the musty odor in foods. 2. Immunoassay evaluation. J. Agric. Food Chem. 2003, 51, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Varelas, V.; Sanvicens, N.; Pilar-Marco, M.; Kintzios, S. Development of a cellular biosensor for the detection of 2,4,6-trichloroanisole (TCA). Talanta 2011, 84, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Kintzios, S. Application of “membrane-engineering” to bioelectric recognition cell sensors for the ultra-sensitive detection of superoxide radical: A novel biosensor principle. Anal. Chim. Acta 2006, 573–574, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Vitsa, K.; Bem, F.; Vassilakos, N.; Perdikaris, A.; Blouhos, P.; Yialouris, C.; Frosyniotis, D.; Anthopoulos, I.; Mangana, O.; et al. Engineering of the membrane of fibroblast cells with virus-specific antibodies: A novel biosensor tool for virus detection. Biosens. Bioelectron. 2008, 24, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Kokla, A.; Blouchos, P.; Livaniou, E.; Zikos, C.; Kakabakos, S.; Petrou, P.; Kintzios, S. Visualization of the membrane engineering concept: Evidence for the specific orientation of electroinserted antibodies and selective binding of target analytes. J. Mol. Recognit. 2013, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.; Pistola, E.; Panagiotopoulos, P.; Bomsel, M.; Alexandropoulos, N.; Bem, F.; Ekonomou, G.; Biselis, J.; Levin, R. Bioelectric recognition assay (BERA). Biosens. Bioelectron. 2001, 16, 325–336. [Google Scholar] [CrossRef]
- Kintzios, S.; Pistola, E.; Konstas, J.; Bem, F.; Matakiadis, T.; Alexandropoulos, N.; Biselis, I.; Levin, R. The application of the bioelectric recognition assay for the detection of human and plant viruses: Definition of operational parameters. Biosens. Bioelectron. 2001, 16, 467–480. [Google Scholar] [CrossRef]
- Apostolou, T.; Moschopoulou, G.; Kolotourou, E.; Kintzios, S. Assessment of in vitro dopamine-neuroblastoma cell interactions with a bioelectric biosensor: Perspective for a novel in vitro functional assay for dopamine agonist/antagonist activity. Talanta 2017, 170, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, T.; Pascual, N.; Marco, M.; Moschos, A.; Petropoulos, A.; Kaltsas, G.; Kintzios, S. Extraction-less, rapid assay for the direct detection of 2,4,6-trichloroanisole (TCA) in cork samples. Talanta 2014, 125, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Pravda, M.; Guilbault, C. Development of a biosensor for the quantitative detection of 2,4,6-trichloroanisole using screen printed electrodes. Anal. Chim. Acta 2003, 484, 15–24. [Google Scholar] [CrossRef]
- Duarte, M.; Lozano-Sanchez, P.; Katakis, I. Copper UPD as non-specific adsorption barrier in electrochemical displacement immunosensors. Biosens. Bioelectron. 2009, 24, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.; Dias, L.; Peres, A.; Sousa, M.; Castro, L.; Veloso, A. Determination of 2,4,6-trichloroanisole by cyclic voltammetry. Procedia Eng. 2012, 47, 1125–1128. [Google Scholar] [CrossRef] [Green Version]
- Peres, A.; Freitas, P.; Dias, L.; Sousa, M.; Castro, L.; Veloso, A. Cyclic voltammetry: A tool to quantify 2,4,6-trichloroanisole in aqueous samples from cork planks boiling industrial process. Talanta 2013, 117, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A.; Lopes, L.; Brito de Barros, R.; Ilharco, L. The problem of 2,4,6-trichloroanisole in cork planks studied by attenuated total reflection infrared spectroscopy: Proof of concept. J. Agric. Food Chem. 2015, 63, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Zalacain, A.; Alonso, G.; Salinas, M. Non-destructive method to determine halophenols and haloanisoles in cork stoppers by headspace sorptive extraction. J. Chromatogr. A 2006, 1114, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Sillero, I.; Cárdenas, S.; Valcárcel, M. Headspace–multicapillary column–ion mobility spectrometry for the direct analysis of 2,4,6-trichloroanisole in wine and cork samples. J. Chromatogr. A 2012, 1265, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Fischer, U. Analysis of cork taint in wine and cork material at olfactory subthreshold levels by solid phase microextraction. J. Agric. Food Chem. 1997, 45, 1995–1997. [Google Scholar] [CrossRef]
- Vestner, J.; de Revel, G.; Krieger-Weber, S.; Rauhut, D.; du Toit, M.; de Villiers, A. Toward automated chromatographic fingerprinting: A non-alignment approach to gas chromatography mass spectrometry data. Anal. Chim. Acta 2016, 911, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Sáenz-González, C.; Pérez-del-Notario, N.; González-Sáiz, J. Microwave assisted extraction combined with dispersive liquid–liquid microextraction as a sensitive sample preparation method for the determination of haloanisoles and halophenols in cork stoppers and oak barrel sawdust. Food Chem. 2012, 132, 2202–2210. [Google Scholar] [CrossRef]





| Biorecognition Element | Assay Concept | Sensitivity (LOD μg L−1) European MRL = 2 μg L−1 | Selectivity | Speed | Quantitative Determination | Sample Pretreatment Required | Ease of Handling | References |
|---|---|---|---|---|---|---|---|---|
| Antibody (polyclonal and monoclonal) | Electrochemical | 0.7 & 0.3 | Yes | <1 h | Yes | Yes | Yes | [32] |
| Antibody (polyclonal) | Electrochemical | 1.5 | Yes | >day | Yes | Yes | Yes | [33] |
| Antibody (monoclonal) | Electrochemical | 0.11 | Yes | >day | No | Yes | Yes | [34] |
| Antibody (polyclonal) | Optical (Microfluidic) | 0.073–28 | Yes | >1 h | Yes | Yes | Yes | [35] |
| Antibody (monoclonal) | Optical | 2 | n/a * | >1 h | Yes | Yes | Yes | [36] |
| Antibody (polyclonal) | QCM-Dissipation monitoring | 0.16 | n/a | <1 h | Yes | Yes | Yes | [38] |
| Antibody (polyclonal) | Electrochemical impedance spectroscopy | 0.000055 | Yes | >1 h | Yes | Yes | Yes | [41] |
| Aptamer | Optical (AuNPs) | 0.009 | Yes | <1 h | Yes | Yes | Yes | [42] |
| Aptamer | Optical (AuNPs) | 20 | Yes | <1 h | Yes | n/a | Yes | [43] |
| Aptamer | PGM | 3.6 | Yes | >1 h | Yes | Yes | Yes | [44] |
| Aptamer | Electrochemical impedance spectroscopy | 0.048–0.16 | Yes | >1 h | Yes | n/a | Yes | [45] |
| - | Optical (CMOS) | 0.002 | n/a | <20 min | Yes | No | Yes | [47] |
| - | Optical (Smartphone) | 2 | n/a | <20 min | Yes | No | Yes | [48] |
| Biorecognition Element | Assay Concept | Sensitivity (LOD μg L−1) | Selectivity | Speed | Quantitative Determination | Sample Pretreatment Required | Ease of Handling | References |
|---|---|---|---|---|---|---|---|---|
| Antibody (polyclonal) | Optical | 2.66 & 2.88 | Yes | >1 d | Yes | Yes | Yes | [67,68] |
| Antibody (polyclonal) | Electrochemical | 0.029 | Yes | >1 d | No | Yes | Yes | [76] |
| Antibody (monoclonal) | Electrochemical | 200 | Yes | >1 h | Yes | Yes | Yes | [77] |
| Cell (membrane engineered with antibodies) | Bioelectric | 0.001 | Yes | <1 h | Yes | No | Yes | [68,75] |
| - | Electrochemical | 0.00031 | Yes | <1 h | Yes | Yes | Yes | [78,79] |
| - | Optical (attenuated total reflection infrared spectroscopy (ATR-IR)) | n/a * | n/a | <1 h | No | No | Yes | [80] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrikou, S.; Kintzios, S. Biosensor-Based Approaches for Detecting Ochratoxin A and 2,4,6-Trichloroanisole in Beverages. Beverages 2018, 4, 24. https://doi.org/10.3390/beverages4010024
Mavrikou S, Kintzios S. Biosensor-Based Approaches for Detecting Ochratoxin A and 2,4,6-Trichloroanisole in Beverages. Beverages. 2018; 4(1):24. https://doi.org/10.3390/beverages4010024
Chicago/Turabian StyleMavrikou, Sophia, and Spyridon Kintzios. 2018. "Biosensor-Based Approaches for Detecting Ochratoxin A and 2,4,6-Trichloroanisole in Beverages" Beverages 4, no. 1: 24. https://doi.org/10.3390/beverages4010024
APA StyleMavrikou, S., & Kintzios, S. (2018). Biosensor-Based Approaches for Detecting Ochratoxin A and 2,4,6-Trichloroanisole in Beverages. Beverages, 4(1), 24. https://doi.org/10.3390/beverages4010024