Physico-Chemical and Sensory Characterization of a Fruit Beer Obtained with the Addition of Cv. Lambrusco Grapes Must
Abstract
:1. Introduction
2. Materials and Methods
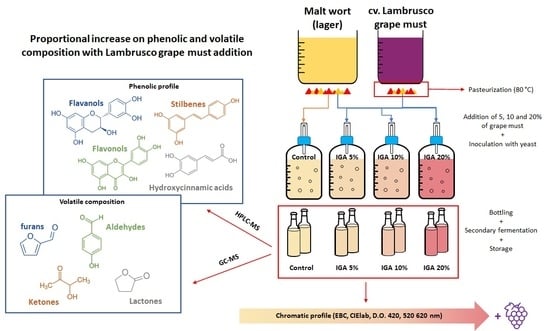
2.1. Sample Preparation
2.2. General Parameters
2.3. Free Phenolics Determination by HPLC-ESI-MS/MS
2.4. Analysis of Volatile Compounds by GC-MS
2.5. Sensory Analysis
- Visive: pale yellow, golden yellow, amber, red ruby, ebony, effervescence, fluidity, foam stability, turbidity, foam compactness.
- Olfactive: malty, red fruits, grapes, yeast, fruity, jam, complexity, floral, citrus, vegetable, dimethyl sulfur (DMS), caramel, tropical fruit, spicy, intensity.
- Gustative: malt, astringency, acid, persistence, smoothness, body, sweet.
2.6. Statistical Analysis
3. Results and Discussion
3.1. General Parameters
3.2. Color Composition of Lambrusco IGA Beers
3.2.1. EBC Color
3.2.2. Spectrophotometric Absorbances at Different Wavelengths: 420, 520 and 620 nm
3.2.3. CIELab Parameters (C, H, L*, a*, b*)
- First, the presence of high-colored Lambrusco musts showed a proportional decrease of L parameter, correlated to lightness or presence of white color (Table 2). As a result, it could be inferred that increases in color intensity led to a lower L component, while reductions of color, as occurred after primary alcoholic fermentation were linked to higher L values.
- As expected, when compared to lager beers, coordinates a* (+ red ⇔ green −) and b* (− blue ⇔ yellow +) displaced towards redder and bluer color with grape must addition, reaching the highest a* (31.59) and the lowest b* values (22.41) in beers added with 20% (Table 2). This demonstrated that the presence of anthocyanins from LGM reduced the yellow component provided by Ctrl pale lager malt (a* = −1.01 and b = 25.33).
- As a consequence, relationships between a* and b* on IGA beers (5, 10 and 20%) shifted the hue (H), generally considered as the “predominant” color, towards reddish hues, proportionally to LGM addition, as showed in Table 2. This result was also confirmed by the color simulation shown in Table 2. Moreover, chroma (C), related to color purity, was also influenced by the presence of grape must, showing the highest (38.74) and the lowest (25.36) values for 20% sample and Ctrl, respectively.
3.3. Phenolics Profiles of Lambrusco IGA Beers
3.4. Volatile Composition of Lambrusco IGA Beers
3.4.1. Alcohols
3.4.2. Esters
3.4.3. Vicinal Diketones (VDKs)
3.4.4. Furans, Pyrans and Lactones
3.4.5. Terpenes
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Class | Compound | rt (min.) | (M-H) | MS2 (−) | (M + H)+ | MS2 (+) |
|---|---|---|---|---|---|---|---|
| 1 | OH-benzoic acids | Gallic acid * | 6.68 | 169 | 125 | n.d. | n.d. |
| 2 | OH-benzoic acids | Protocatechuic acid-O-hexoside (1,2) | 9.90 | 315 | 153, 109 | n.d. | n.d. |
| 4 | OH-cinnamic acids | Caftaric acid (3) | 13.3 | 311 | 177, 149 | n.d. | n.d. |
| 5 | OH-cinnamic acids | 2-S-glutathionyl-caffeoyltartaric acid (3) | 14.7 | 616 | 484, 440, 272 | 618 | 543, 489, 264 |
| 6 | Flavanols | (Epi) Catechin hexoside I (1,2) | 16.8 | 451 | 289, 161 | n.d. | n.d. |
| 8 | OH-cinnamic acids | Coutaric acid (3) | 18.1 | 295 | 163, 149 | n.d. | n.d. |
| 9 | Others | Tyrosol * | 18.5 | 137 | 119 | n.d. | n.d. |
| 10 | Flavanols | (Epi) Catechin hexoside II (1,2) | 18.6 | 451 | 289 | n.d. | n.d. |
| 11 | Flavanols | (Epi) Catechin hexoside III (1,2) | 20.4 | 451 | 289 | n.d. | n.d. |
| 12 | Flavanols | Procyanidin dimer (B1) * | 20.4 | 577 | 425 | 579 | 427, 409, 291 |
| 13 | Flavanols | Procyanidin dimer (3,4) | 20.8 | 577 | 425, 407 | 579 | 427, 409, 453, 291 |
| 14 | OH-cinnamic acids | t-Fertaric acid (3,4) | 21.2 | 325 | 193 | n.d. | n.d. |
| 15 | Flavanols | (+)-Catechin * | 21.7 | 289 | 245, 179, 203 | 291 | 123, 139, 165, 273 |
| 16 | OH-cinnamic acids | Vanillic acid * | 22.2 | 167 | 123, 152, 108 | n.d. | n.d. |
| 17 | OH-cinnamic acids | Feruloylquinic acid isomer (1,2) | 22.3 | 367 | 193, 173 | n.d. | n.d. |
| 18 | OH-cinnamic acids | t-Caffeic acid * | 23.1 | 179 | 135 | n.d. | n.d. |
| 19 | OH-cinnamic acids | c-Fertaric acid (3,4) | 23.1 | 325 | 235, 265 | n.d. | n.d. |
| 20 | OH-benzoic acids | Ethylgallate * | 23.6 | 197 | 182, 153 | n.d. | n.d. |
| 21 | Flavanols | Procyanidins dimer (B2) * | 23.7 | 577 | 425, 407 | 579 | 427, 409, 291 |
| 22 | Flavanols | (+)-Epicatechin * | 24.5 | 289 | 245, 179, 203 | 291 | |
| 23 | Others | Indole lactic acid glucoside (3) | 25.4 | 366 | 204, 186, 142 | n.d. | n.d. |
| 24 | Flavones | Apigenin-C-hexoside-O-hexoside (1,2) | 25.6 | 593 | 312 | 595 | 577, 433, 415, 367 |
| 25 | OH-cinnamic acids | Dihydro-p-coumaric acid (3) | 25.7 | 165 | 147 | n.d. | n.d. |
| 26 | OH-cinnamic acids | Feruloylquinic acid isomer (1,2) | 25.8 | 367 | 193, 173 | n.d. | n.d. |
| 27 | Flavones | Apigenin-C-glycoside-C-pentoside (1,2) | 26.0 | 563 | 443, 473, 383, 545 | 565 | 547, 499, 529, 481, 469 |
| 28 | OH-cinnamic acids | Sinapic acid-O-hexoside (1,2) | 26.1 | 385 | 267 | n.d. | n.d. |
| 29 | Flavonols | Dihydromyricetin 3-O-rhamnoside (4) | 26.4 | 465 | 339, 301, 447 | 467 | 449, 431, 321 |
| 30 | Flavonols | Myricetin glucuronide (4) | 26.5 | 493 | 317 | n.d. | n.d. |
| 31 | OH-cinnamic acids | t-Coumaric acid * | 26.6 | 163 | 119 | n.d. | n.d. |
| 32 | Flavonols | Myricetin-3-glucoside * | 26.6 | 479 | 317 | n.d. | n.d. |
| 33 | Stilbenes | t-Resveratrol glucoside (4) | 28.4 | 389 | 227 | n.d. | n.d. |
| 34 | OH-cinnamic acids | t-Ferulic acid * | 28.4 | 193 | 134, 149, 178 | n.d. | n.d. |
| 35 | Flavones | Apigenin-6C-glucoside (Isovitexin) (1,2) | 28.5 | 431 | 311, 341 | 433 | 367, 415, 337, 313, 283 |
| 36 | OH-cinnamic acids | Sinapic acid (2) | 28.8 | 223 | 208, 164 | n.d. | n.d. |
| 37 | Flavonols | Quercetin-3-glucuronide (4) | 29.0 | 477 | 301 | 479 | 303, 317 |
| 38 | Flavonols | Laricitrin-3-glucoside (4) | 29.2 | 493 | 331 | 495 | 333 |
| 39 | Others | Dihydroquercetin 3-O-rhamnoside (Astilbin) (4) | 29.5 | 449 | 303, 285 | 451 | 415, 433, 315 |
| 40 | Stilbenes | c-Resveratrol glucoside (4) | 31.1 | 389 | 227 | n.d. | n.d. |
| 41 | Stilbenes | t-Resveratrol * | 32.6 | 227 | 185 | n.d. | n.d. |
| 42 | Flavonols | Quercetin * | 33.4 | 301 | 179 | n.d. | n.d. |
| 43 | Isoacids | Iso-α-cohumulone (2) | 36.9 | 347 | 251, 329 | n.d. | n.d. |
| 44 | Isoacids | Iso-α-ad/n-humulone (2) | 37.3 | 361 | 265, 343 | n.d. | n.d. |
| Ctrl | 5% | 10% | 20% | |
|---|---|---|---|---|
| Alcohols | ||||
| Isobutyl alcohol | 2156.02 a | 3039.43 a | 2706.57 a | 2729.83 a |
| n-Butanol | 22.66 b | 43.67 ab | 43.64 ab | 60.62 a |
| 2-penten-4-ol | 21.71 a | 36.62 a | 26.10 a | 28.74 a |
| Isoamyl alcohol | 18,396.81 a | 22,120.79 a | 20,515.96 a | 23,002.98 a |
| 3-methyl-3-Buten-1-ol | 13.33 a | 18.62 a | 16.41 a | 14.85 a |
| 2-Hexanol | 18.79 a | 25.46 a | 19.06 a | 22.41 a |
| 4-Methyl-1-pentanol | 21.12 b | 35.74 a | 31.40 ab | 31.67 ab |
| 2-Buten-1-ol, 3-methyl- | 4.56 a | 4.35 a | 4.88 a | 3.33 a |
| 3-Methyl-1-pentanol | 22.99 b | 41.65 a | 39.93 a | 46.15 a |
| meso-3,4-Hexanediol | 2.28 b | 4.27 ab | 5.58 ab | 19.11 a |
| n-hexanol | 6.33 c | 33.01 b | 46.96 b | 77.94 a |
| 3-ethoxy-1-Propanol | 36.41 a | 40.07 a | 32.05 a | 25.70 a |
| cis-3-Hexene-1-ol | n.d. c | 4.06 bc | 7.71 b | 15.45 a |
| 2-Butoxyethanol | 5.28 a | 4.93 a | 4.39 a | 5.20 a |
| n-Heptanol | 44.87 a | 53.71 a | 53.59 a | 46.26 a |
| 2-Methyl-4-octanol | 7.60 a | 7.64 a | 7.86 a | 10.37 a |
| 3-methyl-2-Octanol | 6.03 a | 6.26 a | 6.91 a | 5.38 b |
| 3-Ethyl-2-heptanol | 7.29 a | 7.66 a | 8.57 a | 7.20 a |
| 2-Ethylhexanol | 9.25 a | 11.45 a | 11.84 a | 10.39 a |
| 3-Nonanol | 20.59 a | 21.37 a | 23.41 a | 18.86 a |
| 2-Nonanol | 3.78 a | 2.71 a | 2.52 a | 1.10 a |
| 2-3-Butanediol | 229.66 a | 223.91 a | 236.56 a | 335.82 a |
| 1-Octanol | 20.60 a | 26.75 a | 24.88 a | 20.39 a |
| 2,3-Butanediol | 54.89 a | 47.58 a | 51.99 a | 84.17 a |
| 1,2-Propanediol | 11.32 a | 7.44 a | 9.31 a | 13.73 a |
| 1-Methoxy-2-butanol | 12.77 a | 12.26 a | 12.26 a | 14.52 a |
| n-decanol | 6.36 a | 9.70 a | 7.63 a | 7.11 a |
| 2,7-dimethyl-4,5-Octanediol | 17.30 a | 21.11 a | 16.72 a | 20.29 a |
| Benzyl alcohol | 10.41 b | 22.89 b | 10.25 b | 51.69 a |
| Phenetyl alcohol | 17,725.51 a | 17,315.69 a | 15,020.83 a | 17,356.69 a |
| 2-Methoxy-4-vinylphenol | 30.53 a | 34.95 a | 23.74 ab | 15.71 b |
| 1-Heptadecanol | 41.36 a | 26.26 a | 31.21 a | 34.44 a |
| total alcohol | 38,988.39 a | 43,311.99 a | 39,060.74 a | 44,142.52 a |
| Fatty Acids | ||||
| Propanoic acid | 12.97 a | 18.39 a | 17.09 a | 15.75 a |
| Isobutyric acid | 112.83 a | 138.58 a | 117.95 a | 95.45 a |
| Butanoic acid | 52.38 a | 82.91 a | 86.69 a | 85.86 a |
| pentanoic acid | 352.55 a | 371.95 a | 308.84 a | 268.45 a |
| Hexanoic acid | 2314.31 a | 2351.84 a | 2070.12 a | 2327.57 a |
| (E)-2-methyl-2-Pentenoic acid | 68.41 a | 59.23 a | 53.70 a | 44.74 a |
| Octanoic acid | 4929.90 a | 5037.27 a | 4369.91 a | 5279.22 a |
| Nonanoic acid | 28.23 ab | 31.94 a | 15.34 b | 25.15 ab |
| Decanoic acid | 2619.96 a | 2427.08 a | 2226.25 a | 2470.79 a |
| 9-Decenoic acid | 344.72 a | 440.73 a | 303.29 a | 384.66 a |
| Benzoic acid | 104.46 a | 128.05 a | 127.18 a | 111.61 a |
| Dodecanoic acid | 643.58 a | 622.25 a | 496.97 a | 454.87 a |
| Phenylacetic acid | 215.05 a | 257.18 a | 221.23 a | 229.06 a |
| Phenylpropionic acid | 29.41 b | 35.59 ab | 42.86 a | 29.03 b |
| Tetradecanoic acid | 47.15 a | 48.55 a | 48.98 a | 32.73 a |
| Pentadecanoic acid | 14.61 a | 17.34 a | 19.79 a | 14.34 a |
| (Z)-Cinnamic acid | 93.62 a | 87.22 a | 70.27 a | 61.57 a |
| Hexadecanoic acid | 259.66 ab | 335.39 a | 351.29 a | 157.31 b |
| Octadecanoic acid | 179.57 a | 179.02 a | 179.86 a | 68.99 a |
| total acids | 12,423.40 a | 12,670.50 a | 11,127.62 a | 12,157.13 a |
| Esters | ||||
| Isoamyl acetate | 109.29 b | 943.53 a | 863.25 a | 483.38 a |
| Ethyl hexanoate | 35.90 b | 379.97 a | 338.96 a | 167.04 a |
| 1-Hexyl acetate | n.d. b | 5.83 a | 7.84 a | 8.86 a |
| Hex-4-enoic acid, ethyl ester | 2.00 ab | 4.29 a | 4.45 a | n.d. b |
| Ethyl lactate | 28.56 b | 55.14 ab | 64.49 ab | 112.68 a |
| Ethyl caprylate | 60.52 b | 440.66 a | 369.12 a | 210.39 a |
| Ethyl 3-hydroxybutyrate | 5.22 b | 7.31 b | 10.78 ab | 23.03 a |
| Ethyl 2-hydroxycaproate | n.d. b | n.d. b | 3.26 a | 3.80 a |
| Ethyl decanoate | 24.24 a | 69.99 a | 82.93 a | 34.99 a |
| Diethyl succinate | n.d. c | 4.32 b | 6.86 ab | 10.55 a |
| ethyl 9-decenoate | 3.74 a | 15.08 a | 12.31 a | 7.82 a |
| β-Phenylethyl acetate | 753.74 a | 873.51 a | 622.85 a | 660.97 a |
| Ethyl dodecanoate | 15.97 a | 15.73 a | 20.27 a | 11.35 a |
| N-Acetylglycine ethyl ester | 8.33 b | 9.87 b | 33.19 a | 43.23 a |
| Ethyl hydrogen succinate | 130.62 c | 237.11 b | 271.71 b | 441.85 a |
| total esters | 1409.52 b | 3062.34 a | 2712.28 a | 2219.95 a |
| Furans | ||||
| Furfural | n.d. b | 2.70 ab | 3.77 a | 6.26 a |
| 2.4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 6.89 b | 17.55 ab | 21.48 a | 18.25 ab |
| 5,5-dimethylfuran-2(5H)-one | 11.53 a | 0.00 b | 0.00 b | 0.00 b |
| Furfuryl alcohol | 60.07 b | 88.17 b | 94.89 ab | 151.60 a |
| 2(3H)-Furanone, 5-ethyldihydro- | n.d. b | n.d. b | 3.41 a | 4.89 a |
| 4H-Pyran-4-one, 3-hydroxy-2-methyl | 25.46 a | 28.61 a | 28.50 a | 22.39 a |
| 2H-Pyran-2,6(3H)-dione | n.d. b | n.d. b | 10.17 a | 13.93 a |
| 2(3H)-Furanone, dihydro-5-pentyl | 8.25 a | 13.68 a | 12.97 a | 12.84 a |
| 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | 10.46 a | 12.71 a | 12.21 a | 11.95 a |
| 5-Hydroxymethyldihydrofuran-2-one | n.d. b | n.d. b | 8.50 a | 53.03 a |
| HMF | n.d. b | 6.78 a | 8.22 a | 9.63 a |
| total furans | 122.66 b | 170.20 ab | 204.12 ab | 304.76 a |
| Ketones | ||||
| 2,3-Pentanedione | n.d. b | n.d. b | n.d. b | 6.37 a |
| Acetoin | 29.95 c | 68.16 b | 40.52 bc | 127.71 a |
| 3-hydroxy-2-pentanone | n.d. c | 4.23 b | 3.78 b | 14.99 a |
| 4-Octanone, 5-hydroxy-2,7-dimethyl- | 42.22 a | 54.66 a | 52.36 a | 61.74 a |
| 4-hydroxy-2-butanone | 359.99 a | 313.60 a | 272.58 a | 238.60 a |
| 3-hydroxy-4-phenyl-2-butanone | 32.43 a | 37.58 a | 36.89 a | 40.80 a |
| total ketones | 464.59 a | 474.00 a | 402.35 a | 475.21 a |
| Aldehydes | ||||
| Benzeneacetaldehyde | 158.16 a | 194.44 a | 177.38 a | 206.68 a |
| p-Hydroxybenzaldehyde | n.d. b | 63.14 ab | 67.30 ab | 104.66 a |
| total aldehydes | 158.16 b | 257.58 ab | 244.68 ab | 311.34 a |
| Terpenes | ||||
| Epoxy Linalool oxide | n.d. b | n.d. b | n.d. b | 4.25 a |
| Geranial | 5.25 a | 4.94 a | 3.23 ab | 1.58 b |
| B-citronellol | 2.54 a | 3.13 a | 3.53 a | 2.04 a |
| (+)-1-Menthene | n.d. c | n.d. c | 2.13 b | 5.75 a |
| Farnesol | 22.32 a | 22.47 a | 20.10 a | 17.85 a |
| total terpenes | 30.11 a | 30.54 a | 28.99 a | 31.47 a |
| Lactones | ||||
| Butyrolactone | 15.63 c | 29.68 bc | 39.10 b | 67.50 a |
| y-Dodecalactone | 10.62 a | 10.57 a | 11.89 a | 5.64 a |
| total lactones | 26.25 c | 40.25 b | 50.99 ab | 73.14 a |
| Others | ||||
| Scyllo-inositol | n.d. c | 2.50 b | 3.85 ab | 7.98 a |
| Methionol | 736.93 a | 838.61 a | 684.14 ab | 941.54 a |
| Acetylpyrrole | 13.70 a | 16.20 a | 13.83 a | 12.59 a |
| Acetovanillone | n.d. c | 13.13 b | 14.70 b | 27.80 a |
References
- Pavsler, A.; Buiatti, S. Lager beer. Beer Health Dis. Prev. 2008, 31–43. [Google Scholar] [CrossRef]
- Gatza, P.; Swersey, C.; Skypeck, C.; Rabin, D. BJCP Beer Style Guidelines 2015. Brew. Assoc. 2017, 47, 556. [Google Scholar]
- Ferrari, A.M.; Pini, M.; Sassi, D.; Zerazion, E.; Neri, P. Effects of grape quality on the environmental profile of an Italian vineyard for Lambrusco red wine production. J. Clean. Prod. 2018, 172, 3760–3769. [Google Scholar] [CrossRef]
- Catena, M. Il Lambrusco: La Lunga Storia di un Vino di Successo; Consorzio Marchio Storico dei Lambruschi Modenesi: Modena, Italy, 2015. [Google Scholar]
- Vasile Simone, G.; Montevecchi, G.; Masino, F.; Matrella, V.; Imazio, S.A.; Antonelli, A.; Bignami, C. Ampelographic and chemical characterization of Reggio Emilia and Modena (northern Italy) grapes for two traditional seasonings: “Saba” and “agresto”. J. Sci. Food Agric. 2013, 93, 3502–3511. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2013; ISBN 9791091799188.
- Glories, Y. Recherches sur la Matière Colorante des Vins Rouges. Ph.D. Thesis, Université de Bordeaux II, Bordeaux, France, 1879. [Google Scholar]
- Del Álamo, M.; Casado, L.; Hernández, V.; Jiménez, J.J. Determination of free molecular phenolics and catechins in wine by solid phase extraction on polymeric cartridges and liquid chromatography with diode array detection. J. Chromatogr. A 2004, 1049, 97–105. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Bellachioma, A.; Versari, A.; Riponi, C. Changes in phenolic composition of red wines aged in cherry wood. LWT Food Sci. Technol. 2015, 60, 977–984. [Google Scholar] [CrossRef]
- Moio, L.; Chambellant, E.; Lesschaeve, I.; Issanchou, S.; Schlich, P.E.P. Production of representative wine extracts for chemical and olfactory analysis. Ital. J. Food Sci. 1995, 7, 265–278. [Google Scholar]
- Castro-Marin, A.; Buglia, A.G.; Riponi, C.; Chinnici, F. Volatile and fixed composition of sulphite-free white wines obtained after fermentation in the presence of chitosan. LWT Food Sci. Technol. 2018, 93, 174–180. [Google Scholar] [CrossRef]
- Donadini, G.; Fumi, M.D.; Kordialik-Bogacka, E.; Maggi, L.; Lambri, M.; Sckokai, P. Consumer interest in specialty beers in three European markets. Food Res. Int. 2016, 85, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Donadini, G.; Fumi, M.D.; Lambri, M. A preliminary study investigating consumer preference for cheese and beer pairings. Food Qual. Prefer. 2013, 30, 217–228. [Google Scholar] [CrossRef]
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces cerevisiae var. boulardii: Valuable probiotic starter for craft beer production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef] [Green Version]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Shatunova, S.A.; Petrov, A.S. Production and analysis of non-traditional beer supplemented with sea buckthorn. Agron. Res. 2017, 15, 1831–1845. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M. Influence of kilning temperature on chemical composition of a Greek barley malt and its wort properties. Millenium 2018, 2, 49–58. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Bamforth, C.W. Assessing color quality of beer. ACS Symp. Ser. 2008, 983, 192–202. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Riponi, C. Efficacy of Chitosan in Inhibiting the Oxidation of (+)-Catechin in White Wine Model Solutions. J. Agric. Food Chem. 2014, 5, 9868–9875. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [Green Version]
- Shellhammer, T.H. Beer: A quality perspective. In Handbook of Alcoholic Beverages Series; Academic Press: Cambridge, MA, USA, 2009; pp. 213–227. [Google Scholar]
- García-Marino, M.; Escudero-Gilete, M.L.; Heredia, F.J.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C. Color-copigmentation study by tristimulus colorimetry (CIELAB) in red wines obtained from Tempranillo and Graciano varieties. Food Res. Int. 2013, 51, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2010, 30, 491–509. [Google Scholar] [CrossRef]
- Cortese, M.; Gigliobianco, M.R.; Peregrina, D.V.; Sagratini, G.; Censi, R.; Di Martino, P. Quantification of phenolic compounds in different types of crafts beers, worts, starting and spent ingredients by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1612, 460622. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Optimization of a liquid chromatography method based on simultaneous electrospray ionization mass spectrometric and ultraviolet photodiode array detection for analysis of flavonoid glycosides. Rapid Commun. Mass Spectrom. 2002, 16, 2341–2348. [Google Scholar] [CrossRef]
- Callemien, D.; Collin, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—A review. Food Rev. Int. 2010, 26, 1–84. [Google Scholar] [CrossRef]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. ISRN Spectrosc. 2013, 2013, 1–45. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Lentz, M. The impact of simple phenolic compounds on beer aroma and flavor. Fermentation 2018, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Younis, O.S.; Stewart, G.G. Sugar uptake and subsequent ester and higher alcohol production by Saccharomyces cerevisiae. J. Inst. Brew. 1998, 104, 255–264. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogerus, K.; Gibson, B.R. 125th anniversary review: Diacetyl and its control during brewery fermentation. J. Inst. Brew. 2013, 119, 86–97. [Google Scholar] [CrossRef]
- Cyr, N.; Blanchette, M.; Price, S.P.; Sheppard, J.D. Vicinal diketone production and amino acid uptake by two active dry lager yeasts during beer fermentation. J. Am. Soc. Brew. Chem. 2007, 65, 138–144. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Kanauchi, M. Enzymology of vicinal diketone reduction in brewer’s yeast. J. Inst. Brew. 2004, 110, 83–93. [Google Scholar] [CrossRef]
- Stewart, G.G. The production of secondary metabolites with flavour potential during brewing and distilling wort fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.J. Thermal Treatment: Hydroxymethylfurfural (HMF) and Related Compounds. Process. Food Toxic. Occur. Form. Mitig. Health Risks 2008, 135–174. [Google Scholar] [CrossRef]
- Anese, M.; Suman, M. Mitigation strategies of furan and 5-hydroxymethylfurfural in food. Food Res. Int. 2013, 51, 257–264. [Google Scholar] [CrossRef]
- Parker, D.K. Beer: Production, Sensory Characteristics and Sensory Analysis; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Vanderhaegen, B.; Neven, H.; Verstrepen, K.J.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Influence of the brewing process on furfuryl ethyl ether formation during beer aging. J. Agric. Food Chem. 2004, 52, 6755–6764. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. S. Afr. J. Enol. Vitic. 2017, 4, 49–58. [Google Scholar] [CrossRef]
- Cheiran, K.P.; Raimundo, V.P.; Manfroi, V.; Anzanello, M.J.; Kahmann, A.; Rodrigues, E.; Frazzon, J. Simultaneous identification of low-molecular weight phenolic and nitrogen compounds in craft beers by HPLC-ESI-MS/MS. Food Chem. 2019, 286, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Benucci, I.; Antonacci, D.; Di Luccia, A.; Esti, M. HPLC-DAD-MS/MS characterization of phenolic compounds in white wine stored without added sulfite. Food Res. Int. 2014, 66, 207–215. [Google Scholar] [CrossRef]




| pH | TA | ABV % | °Brix | Extract | TPI | Astringency (%) | |
|---|---|---|---|---|---|---|---|
| Pre-ferm | |||||||
| Grape must | 3.40 e | 14.92 a | - | 18.5 a | 1.075 # | 1845 a | - |
| Beer wort | 6.01 a | 0.77 e | - | 8.9 e | 9.02 d | 286 b | - |
| 5% | 4.43 b | 1.25 d | - | 9.3 d | 9.51 c | 304 c | - |
| 10% | 3.98 c | 2.10 c | - | 9.8 c | 9.99 b | 321 d | - |
| 20% | 3.67 d | 3.57 b | - | 10.9 b | 10.56 a | 361 e | - |
| 1st Ferm | |||||||
| Ctrl | 3.95 a | 2.45 d | n.d. | - | 3.07 a | 275 a | n.d. |
| 5% | 3.77 b | 3.00 c | n.d. | - | 3.07 a | 291 b | n.d. |
| 10% | 3.68 c | 3.67 b | n.d. | - | 3.07 a | 304 c | n.d. |
| 20% | 3.59 d | 4.62 a | n.d. | - | 3.07 a | 345 d | n.d. |
| Storage | |||||||
| Ctrl | 3.91 a | 2.43 d | 4.70 d | - | 1.21 b | 274 a | 3.99 d |
| 5% | 3.74 b | 2.80 c | 5.04 c | - | 1.20 a | 291 b | 4.59 c |
| 10% | 3.67 c | 3.13 b | 5.37 b | - | 1.12 a | 304 c | 5.89 b |
| 20% | 3.59 d | 4.87 a | 6.12 a | - | 1.13 a | 338 d | 6.30 a |
| 420 nm | 520 nm | 620 nm | EBC | L* | a* | b* | C | H | Color * | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ferm | ||||||||||
| Ctrl | 0.451 d | 0.128 d | 0.055 c | 9.73 d | 90.97 a | −0.25 d | 26.63 a | 26.63 b | 90.55 a | |
| 5% | 0.472 c | 0.163 c | 0.055 c | 10.03 c | 88.23 b | 2.77 c | 22.08 b | 22.25 c | 82.84 b | |
| 10% | 0.505 b | 0.293 b | 0.078 b | 11.13 b | 82.69 c | 12.00 b | 19.02 c | 22.51 c | 57.79 c | |
| 20% | 0.705 a | 0.814 a | 0.151 a | 16.4 a | 64.82 d | 38.71 a | 14.85 d | 41.47 a | 20.99 d | |
| 1st Ferm | ||||||||||
| Ctrl | 0.392 d | 0.085 d | 0.024 d | 8.31 d | 91.68 a | −1.37 d | 24.15 a | 24.08 b | 93.27 a | |
| 5% | 0.429 c | 0.154 c | 0.039 c | 9.34 c | 87.87 ab | 3.87 c | 23.12 a | 23.45 b | 80.63 b | |
| 10% | 0.469 b | 0.243 b | 0.055 b | 10.35 b | 80.28 b | 9.93 b | 21.15 b | 23.37 b | 64.86 c | |
| 20% | 0.573 a | 0.506 a | 0.089 a | 13.18 a | 70.80 c | 26.37 a | 18.78 c | 32.37 a | 35.46 d | |
| Storage | ||||||||||
| Ctrl | 0.446 b | 0.102 d | 0.03 c | 9.15 d | 92.83 a | −1.01 d | 25.33 a | 25.35 b | 92.30 a | |
| 5% | 0.464 b | 0.192 c | 0.051 b | 10.5 c | 86.07 b | 5.25 c | 24.38 ab | 25.01 b | 77.84 b | |
| 10% | 0.513 b | 0.287 b | 0.064 b | 11.45 b | 82.70 b | 12.63 b | 23.02 b | 26.23 b | 61.26 c | |
| 20% | 0.675 a | 0.623 a | 0.113 a | 15.8 a | 69.27 c | 31.59 a | 22.41 b | 38.74 a | 35.33 d |
| Compound | Control | 5% | 10% | 20% |
|---|---|---|---|---|
| Hydroxybenzoic acids | ||||
| Gallic acid | n.d. b | n.d. b | 0.02 ab | 0.06 a |
| Protocatechuic acid-O-hexoside | n.d. b | 0.03 b | 0.10 ab | 0.27 a |
| Ethylgallate | 0.13 | 0.34 | 0.56 | 0.51 |
| Sum Hydroxybenzoic acids | 0.13 b | 0.36 b | 0.68 ab | 0.84 a |
| Hydroxycinnamic acids | ||||
| Caftaric acid | n.d. c | 0.56 c | 4.62 b | 11.06 a |
| 2-S-glutathionyl-caffeoyltartaric acid | n.d. b | n.d. b | n.d. b | 0.06 a |
| t-Coutaric acid | n.d. d | 2.09 c | 17.01 b | 34.52 a |
| t-Fertaric acid | n.d. d | 0.70 c | 5.27 b | 18.11 a |
| Vanillic acid | 0.25 | 0.21 | 0.27 | 0.30 |
| Feruloylquinic acid isomer | 2.19 a | 1.21 b | 1.96 ab | 1.84 ab |
| t-Caffeic acid | 0.10 c | 0.24 c | 0.53 b | 1.20 a |
| c-Fertaric acid | n.d. d | 0.54 c | 0.97 b | 1.73 a |
| Dihydro-p-coumaric acid | n.d. c | 2.06 b | 5.12 ab | 5.36 a |
| Feruloylquinic acid isomer | 1.10 | 0.79 | 1.18 | 1.18 |
| Sinapic acid-O-hexoside | 0.88 a | 0.62 b | 0.79 ab | 0.72 ab |
| t-Coumaric acid | 1.00 | 0.77 | 0.86 | 1.00 |
| t-Ferulic acid | 2.73 a | 2.46 b | 2.60 ab | 2.43 b |
| Sinapic acid | 0.51 a | 0.30 b | 0.40 b | 0.34 b |
| Sum Hydroxycinnamic acids | 8.77 c | 12.57 c | 41.59 b | 79.84 a |
| Flavanols | ||||
| (Epi) Catechin hexoside I | 0.13 b | 0.23 b | 0.97 a | 1.11 a |
| (Epi) Catechin hexoside II | 0.21 a | 0.01 b | n.d. b | n.d. b |
| (Epi) Catechin hexoside III | 0.16 a | 0.08 b | 0.06 b | 0.02 b |
| Procyanidin dimer (B1) | n.d. d | 0.20 c | 0.73 b | 1.45 a |
| Procyanidin dimer | 0.10 b | 0.10 b | 0.35 ab | 0.55 a |
| (+)-Catechin | 1.92 d | 3.31 c | 6.02 b | 10.89 a |
| Procyanidin dimer (B2) | n.d. c | 0.07 c | 0.23 b | 0.50 a |
| (+)-Epicatechin | n.d. d | 0.48 c | 1.16 b | 2.82 a |
| Sum Flavanols | 2.51 c | 4.47 bc | 9.51 b | 17.34 a |
| Flavones | ||||
| Apigenin-C-hexoside-O-hexoside | 0.35 | 0.23 | 0.26 | 0.23 |
| Apigenin-C-hexoside-C-pentoside | 0.51 a | 0.36 bc | 0.46 ab | 0.28 c |
| Apigenin C-hexoside (Isovitexin) | 0.41 a | 0.33 a | 0.40 a | n.d. b |
| Sum Flavones | 1.27 a | 0.91 ab | 1.12 a | 0.51 b |
| Flavonols | ||||
| Myricetin 3-glucoside | n.d. c | 0.15 b | 0.42 ab | 1.01 a |
| Quercetin-3-glucuronide | n.d. d | 0.33 c | 1.15 b | 2.55 a |
| Laricitrin 3-glucoside | n.d. c | n.d. c | 0.09 b | 0.20 a |
| Quercetin | n.d. c | 0.14 c | 6.50 a | 1.88 b |
| Sum Flavonols | 0.00 c | 0.62 b | 8.15 a | 5.63 a |
| Stilbenes | ||||
| t-Resveratrol glucoside | n.d. b | n.d. b | 0.08 b | 1.05 a |
| c-Resveratrol glucoside | n.d. c | n.d. c | 0.12 b | 0.40 a |
| t-Resveratrol | n.d. c | 0.21 b | 0.41 ab | 0.71 a |
| Sum Stilbenes | 0.00 d | 0.21 c | 0.60 b | 2.17 a |
| Others | ||||
| Tyrosol | 3.53 a | 2.58 ab | 2.89 ab | 2.20 b |
| Indole lactic acid glucoside | n.d. d | 0.33 c | 0.81 b | 1.52 a |
| Dihydroquercetin 3-O-rhamnoside (Astilbin) | n.d. d | 0.78 c | 1.91 b | 3.58 a |
| Dihydromyricetin 3-O-rhamnoside | n.d. d | 1.28 c | 2.57 b | 4.26 a |
| Sum Others | 3.53 d | 4.97 c | 8.17 b | 11.57 a |
| Sum phenolics | 16.21 c | 24.11 c | 69.83 b | 117.89 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Marin, A.; Baris, F.; Romanini, E.; Lambri, M.; Montevecchi, G.; Chinnici, F. Physico-Chemical and Sensory Characterization of a Fruit Beer Obtained with the Addition of Cv. Lambrusco Grapes Must. Beverages 2021, 7, 34. https://doi.org/10.3390/beverages7020034
Castro Marin A, Baris F, Romanini E, Lambri M, Montevecchi G, Chinnici F. Physico-Chemical and Sensory Characterization of a Fruit Beer Obtained with the Addition of Cv. Lambrusco Grapes Must. Beverages. 2021; 7(2):34. https://doi.org/10.3390/beverages7020034
Chicago/Turabian StyleCastro Marin, Antonio, Federico Baris, Elia Romanini, Milena Lambri, Giuseppe Montevecchi, and Fabio Chinnici. 2021. "Physico-Chemical and Sensory Characterization of a Fruit Beer Obtained with the Addition of Cv. Lambrusco Grapes Must" Beverages 7, no. 2: 34. https://doi.org/10.3390/beverages7020034
APA StyleCastro Marin, A., Baris, F., Romanini, E., Lambri, M., Montevecchi, G., & Chinnici, F. (2021). Physico-Chemical and Sensory Characterization of a Fruit Beer Obtained with the Addition of Cv. Lambrusco Grapes Must. Beverages, 7(2), 34. https://doi.org/10.3390/beverages7020034