The Association of Fasting Glucose, Insulin, and C-Peptide, with 19-Year Incidence of Coronary Heart Disease in Older Japanese-American Men; the Honolulu Heart Program
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
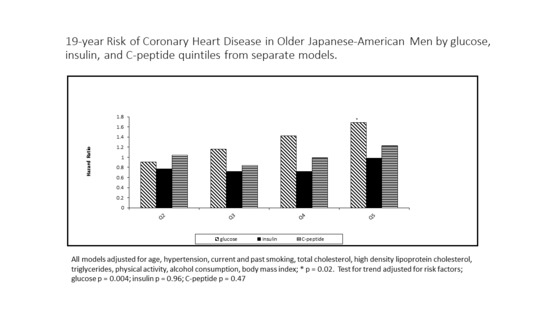
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| CHD | Coronary heart disease |
| HHP | Honolulu Heart Program |
| IGT | Impaired glucose tolerance |
| DM | Diabetes mellitus |
| MI | Myocardial infarction |
| FBG | Fasting Glucose |
| BMI | Body mass index |
| HDL-C | HDL cholesterol |
| ADA | American Diabetes Association |
| Journal Subject Codes | 3, 4, 190, 192 |
References
- Quadros, A.S.; Sarmento-Leite, R.; Bertoluci, M.; Duro, K.; Schmidt, A.; De Lucca, G., Jr.; Schaan, B.D. Angiographic coronary artery disease is associated with progressively higher levels of fasting plasma glucose. Diabetes Res. Clin. Pract. 2007, 75, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Muhlestein, J.B.; Anderson, J.L.; Horne, B.D.; Lavasani, F.; Allen Maycock, C.A.; Bair, T.L.; Pearson, R.R.; Carlquist, J.F. Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention. Am. Heart J. 2003, 146, 351–358. [Google Scholar] [CrossRef]
- Coutinho, M.; Gerstein, H.C.; Wang, Y.; Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999, 22, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Lau, N.; Burchfiel, C.M.; Abbott, R.D.; Sharp, D.S.; Yano, K.; Curb, J.D. Glucose intolerance and 23-year risk of coronary heart disease and total mortality: The Honolulu Heart Program. Diabetes Care 1999, 22, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Surdacki, A.; Stochmal, E.; Szurkowska, M.; Bode-Boger, S.M.; Martens-Lobenhoffer, J.; Stochmal, A.; Klecha, A.; Kawecka-Jaszcz, K.; Dubiel, J.S.; Huszno, B.; et al. Nontraditional atherosclerotic risk factors and extent of coronary atherosclerosis in patients with combined impaired fasting glucose and impaired glucose tolerance. Metab. Clin. Exp. 2007, 56, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, K.S.; Rubenstein, A.H. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes 1984, 33, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, K.; Frank, B.; Pugh, W.; Addis, A.; Karrison, T.; Meier, P.; Tager, H.; Rubenstein, A. The limitations to and valid use of C-peptide as a marker of the secretion of insulin. Diabetes 1986, 35, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, L.; Zheng, L. Association between serum C-peptide as a risk factor for cardiovascular disease and high-density lipoprotein cholesterol levels in nondiabetic individuals. PLoS ONE 2015, 10, e112281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, D.; Meng, L.; Enwer, G. Serum C-peptide as a key contributor to lipid-related residual cardiovascular risk in the elderly. Arch. Gerontol. Geriatr. 2017, 73, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Silbernagel, G.; Brandenburg, V.; Burgmaier, M.; Kleber, M.E.; Grammer, T.B.; Winkelmann, B.R.; Boehm, B.O.; Marz, W. C-peptide levels are associated with mortality and cardiovascular mortality in patients undergoing angiography: The LURIC study. Diabetes Care 2013, 36, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Worth, R.M.; Kagan, A. Ascertainment of men of Japanese ancestry in Hawaii through World War II Selective Service registration. J. Chronic Dis. 1970, 23, 389–397. [Google Scholar] [CrossRef]
- Yano, K.; Reed, D.M.; McGee, D.L. Ten-year incidence of coronary heart disease in the Honolulu Heart Program. Relationship to biologic and lifestyle characteristics. Am. J. Epidemiol. 1984, 119, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.P.; Doyle, J.T.; Gordon, T.; Hames, C.G.; Hjortland, M.C.; Hulley, S.B.; Kagan, A.; Zukel, W.J. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977, 55, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, G.G.; Gulbrandsen, C.L.; Kagan, A. Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N. Engl. J. Med. 1976, 294, 293–298. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar]
- Lautamaki, R.; Airaksinen, K.E.; Seppanen, M.; Toikka, J.; Harkonen, R.; Luotolahti, M.; Borra, R.; Sundell, J.; Knuuti, J.; Nuutila, P. Insulin improves myocardial blood flow in patients with type 2 diabetes and coronary artery disease. Diabetes 2006, 55, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.G.; Fontaine, M.J.; Rogers, J. C-peptide: Much more than a byproduct of insulin biosynthesis. Pancreas 2004, 29, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Gentile, L.; Castiglione, A.; Prandi, V.; Canil, S.; Ghigo, E.; Ciccone, G. C-peptide and the risk for incident complications and mortality in type 2 diabetic patients: A retrospective cohort study after a 14-year follow-up. Eur. J. Endocrinol. 2012, 167, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Taveira, T.H.; Choudhary, G.; Whitlatch, H.; Wu, W.C. Fasting serum C-peptide levels predict cardiovascular and overall death in nondiabetic adults. J. Am. Heart Assoc. 2012, 1, e003152. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Min, K.B. Serum C-peptide levels and risk of death among adults without diabetes mellitus. Can. Med. Assoc. J. 2013, 185, E402–E408. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Fujimoto, W.Y.; Kahn, S.E.; Weigle, D.S.; McNeely, M.J.; Leonetti, D.L.; Shofer, J.B.; Boyko, E.J. Insulin, C-peptide, and leptin concentrations predict increased visceral adiposity at 5- and 10-year follow-ups in nondiabetic Japanese Americans. Diabetes 2005, 54, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Arcavi, L.; Behar, S.; Caspi, A.; Reshef, N.; Boyko, V.; Knobler, H. High fasting glucose levels as a predictor of worse clinical outcome in patients with coronary artery disease: Results from the Bezafibrate Infarction Prevention (BIP) study. Am. Heart J. 2004, 147, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.; Boyko, V.; Benderly, M.; Mandelzweig, L.; Graff, E.; Reicher-Reiss, H.; Schneider, H.; Shotan, A.; Balkin, J.; Brunner, D.; et al. Asymptomatic hyperglycemia in coronary heart disease: Frequency and associated lipid and lipoprotein levels in the bezafibrate infarction prevention (BIP) register. J. Cardiovasc. Risk 1995, 2, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, M.; Ryden, L.; Ohrvik, J.; Bartnik, M.; Malmberg, K.; Scholte Op Reimer, W.; Simoons, M.L. Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: A report from the Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2006, 27, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Halter, J.B.; Musi, N.; McFarland Horne, F.; Crandall, J.P.; Goldberg, A.; Harkless, L.; Hazzard, W.R.; Huang, E.S.; Kirkman, M.S.; Plutzky, J.; et al. Diabetes and cardiovascular disease in older adults: Current status and future directions. Diabetes 2014, 63, 2578–2589. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Curb, J.D.; Burchfiel, C.M.; Huang, B.; Sharp, D.S.; Lu, G.Y.; Fujimoto, W.; Yano, K. Impaired glucose tolerance, diabetes, and cardiovascular disease risk factor profiles in the elderly. The Honolulu Heart Program. Diabetes Care 1996, 19, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Curb, J.D.; Rodriguez, B.L.; Burchfiel, C.M.; Abbott, R.D.; Chiu, D.; Yano, K. Sudden death, impaired glucose tolerance, and diabetes in Japanese American men. Circulation 1995, 91, 2591–2595. [Google Scholar] [CrossRef] [PubMed]
- Burchfiel, C.M.; Curb, J.D.; Rodriguez, B.L.; Abbott, R.D.; Chiu, D.; Yano, K. Glucose intolerance and 22-year stroke incidence. The Honolulu Heart Program. Stroke 1994, 25, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, W.Y.; Bergstrom, R.W.; Boyko, E.J.; Chen, K.W.; Leonetti, D.L.; Newell-Morris, L.; Shofer, J.B.; Wahl, P.W. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care 1999, 22, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Abbott, R.D.; Fujimoto, W.; Waitzfelder, B.; Chen, R.; Masaki, K.; Schatz, I.; Petrovitch, H.; Ross, W.; Yano, K.; et al. The American Diabetes Association and World Health Organization classifications for diabetes: Their impact on diabetes prevalence and total and cardiovascular disease mortality in elderly Japanese-American men. Diabetes Care 2002, 25, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Saab, K.R.; Kendrick, J.; Yracheta, J.M.; Lanaspa, M.A.; Pollard, M.; Johnson, R.J. New Insights on the Risk for Cardiovascular Disease in African Americans: The Role of Added Sugars. J. Am. Soc. Nephrol. 2015, 26, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Bergoderi, M.; Goel, K.; Murad, M.H.; Allison, T.; Somers, V.K.; Erwin, P.J.; Sochor, O.; Lopez-Jimenez, F. Cardiovascular mortality in Hispanics compared to non-Hispanic whites: A systematic review and meta-analysis of the Hispanic paradox. Eur. J. Intern. Med. 2013, 24, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Medina-Inojosa, J.; Jean, N.; Cortes-Bergoderi, M.; Lopez-Jimenez, F. The Hispanic paradox in cardiovascular disease and total mortality. Prog. Cardiovasc. Dis. 2014, 57, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Ewing, S.K.; Liu, K.; Blaha, M.J.; Dave, S.S.; Qureshi, F.; Kandula, N.R. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: The MASALA and MESA studies. Diabetes Care 2014, 37, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Bellary, S.; O’Hare, J.P.; Raymond, N.T.; Mughal, S.; Hanif, W.M.; Jones, A.; Kumar, S.; Barnett, A.H. Premature cardiovascular events and mortality in south Asians with type 2 diabetes in the United Kingdom Asian Diabetes Study—Effect of ethnicity on risk. Curr. Med. Res. Opin. 2010, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.O.; Govan, L.; Petrie, J.R.; Ghouri, N.; Leese, G.; Fischbacher, C.; Colhoun, H.; Philip, S.; Wild, S.; McCrimmon, R.; et al. Ethnicity and risk of cardiovascular disease (CVD): 4.8 year follow-up of patients with type 2 diabetes living in Scotland. Diabetologia 2015, 58, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Lawes, C.M.; Vander Hoorn, S.; Murray, C.J.; Ezzati, M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: Comparative risk assessment. Lancet 2006, 368, 1651–1659. [Google Scholar] [CrossRef]


| Variable | Q1 * | Q2 * | Q3 * | Q4 * | Q5 * | p Value |
|---|---|---|---|---|---|---|
| N | 235 | 231 | 245 | 213 | 175 | |
| Average Age (years) | 67.46 | 67.87 | 67.62 | 67.5 | 67.96 | |
| Total cholesterol (mg/dL) | 205.4 | 209.8 | 215.1 | 214.2 | 214.8 | <0.01 |
| HDL-C † (mg/dL) | 50.28 | 48.61 | 49.04 | 47.79 | 43.30 | <0.01 |
| Triglycerides (mg/dL) | 133.8 | 146.5 | 163.6 | 176.1 | 257.8 | <0.01 |
| Treatment for dyslipidemia (%) | 1 | 1 | 2 | 1 | 0 | 0.45 |
| Body mass index (Kg/m2) | 22.56 | 23.03 | 23.65 | 23.93 | 24.79 | <0.01 |
| Mean systolic blood pressure (mmHg) | 134.4 | 136.9 | 138.8 | 141.5 | 143.3 | <0.01 |
| Mean diastolic blood pressure (mmHg) | 79.91 | 80 | 81.12 | 82.85 | 83.57 | <0.01 |
| History or treatment of hypertension (%) | 23 | 35 | 35 | 48 | 46 | <0.01 |
| Current Smoking (%) | 26 | 22 | 22 | 14 | 17 | <0.01 |
| Past Smoking (%) | 40 | 44 | 45 | 45 | 49 | 0.08 |
| Alcohol (mL/week) | 79.54 | 98.76 | 113.84 | 104.68 | 128.33 | <0.01 |
| Variable | Q1 * | Q2 * | Q3 * | Q4 * | Q5 * |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 94.12 | 101.18 | 106.4 | 113.46 | 143.28 |
| Glucose range (mg/dL) | 73.00–98.00 | 99.00–103.00 | 104.00–109.00 | 110.00–119.00 | 120.00–417.00 |
| Insulin (µU/mL) | 5.65 | 8.74 | 11.47 | 15.16 | 25.51 |
| C-peptide (ng/mL) | 0.76 | 1.16 | 1.54 | 1.93 | 2.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, N.; Chen, R.; Curb, J.D.; Willcox, B.; Rodriguez, B.L. The Association of Fasting Glucose, Insulin, and C-Peptide, with 19-Year Incidence of Coronary Heart Disease in Older Japanese-American Men; the Honolulu Heart Program. Geriatrics 2018, 3, 22. https://doi.org/10.3390/geriatrics3020022
Wahab N, Chen R, Curb JD, Willcox B, Rodriguez BL. The Association of Fasting Glucose, Insulin, and C-Peptide, with 19-Year Incidence of Coronary Heart Disease in Older Japanese-American Men; the Honolulu Heart Program. Geriatrics. 2018; 3(2):22. https://doi.org/10.3390/geriatrics3020022
Chicago/Turabian StyleWahab, Nazneem, Randi Chen, Jess David Curb, Bradley Willcox, and Beatriz L. Rodriguez. 2018. "The Association of Fasting Glucose, Insulin, and C-Peptide, with 19-Year Incidence of Coronary Heart Disease in Older Japanese-American Men; the Honolulu Heart Program" Geriatrics 3, no. 2: 22. https://doi.org/10.3390/geriatrics3020022
APA StyleWahab, N., Chen, R., Curb, J. D., Willcox, B., & Rodriguez, B. L. (2018). The Association of Fasting Glucose, Insulin, and C-Peptide, with 19-Year Incidence of Coronary Heart Disease in Older Japanese-American Men; the Honolulu Heart Program. Geriatrics, 3(2), 22. https://doi.org/10.3390/geriatrics3020022