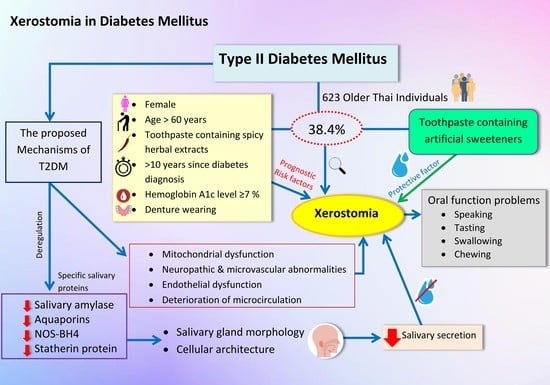
The Prevalence of Xerostomia in Older Thai Individuals with Type II Diabetes Mellitus and Its Association with Type of Toothpaste and Oral Functions: A Cross-Sectional Study Using Questionnaires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Inclusion Criteria
2.2. Assessment of Xerostomia and Questionnaire Survey
2.3. Measurement of Salivary Flow Rates and Oral Dryness Examinations
2.4. Statistical Analysis
3. Results
3.1. Prevalence of Xerostomia
3.2. Xerostomia in Relation to Diabetic Status and Toothpaste
3.3. The Prevalence of Xerostomia Related to Oral Function Problems
3.4. Factors Associated with Xerostomia
4. Discussion
4.1. Xerostomia
4.2. Type of Toothpaste
4.3. Oral Function Problems
4.4. The Association between Diabetes, Xerostomia, Type of Toothpaste, and Oral Function Problems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, P.; Suarez-Durall, P.; Mulligan, R. Dry mouth: A critical topic for older adult patients. J. Prosthodont. Res. 2015, 59, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, L.M.; Thomson, W.M. Xerostomia: Its Prevalence and Associations in the Adult Australian Population. Aust. Dent. J. 2020, 65, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Kuthasema, P.; Putwatana, P.; Junhavat, D. Experience of Xerostomia, Management, and Outcomes in Patients with Head and Neck Cancer post Radiation. Rama Nurs. J. 2010, 16, 40–53. (In Thai) [Google Scholar]
- Ogawa, M.; Tsuji, T. Functional salivary gland regeneration as the next generation of organ replacement regenerative therapy. Odontology 2015, 103, 248–257. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 10TH edition, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; pp. 2–10. [Google Scholar]
- Ohara, Y.; Iwasaki, M.; Shirobe, M.; Kawai, H.; Edahiro, A.; Motokawa, K.; Fujiwara, Y.; Kim, H.; Ihara, K.; Obuchi, S.; et al. Xerostomia as a key predictor of physical frailty among community-dwelling older adults in Japan: A five-year prospective cohort study from The Otassha Study. Arch. Gerontol. Geriatr. 2022, 99, 104608. [Google Scholar] [CrossRef]
- Pérez-González, A.; Suárez-Quintanilla, J.A.; Otero-Rey, E.; Blanco-Carrión, A.; Gómez-García, F.J.; Gándara-Vila, P.; Martín-Biedma, B.; Pérez-Sayáns, M. Association between xerostomia, oral and general health, and obesity in adults. A cross-sectional pilot study. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, 762–769. [Google Scholar] [CrossRef]
- de Carvalho, H.N.; Dos Santos, Y.L.; de Macedo Bernardino, Í.; de Lima, K.C.; Granville-Garcia, A.F.; de Brito Costa, E.M.M. Accuracy of a questionnaire on xerostomia as a screening tool for hyposalivation. Int. Dent. J. 2020, 70, 427–434. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.C.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Kunon, N.; Kaewplung, O. Comparison of chewing ability of mandibular implant-retained overdenture patients using the subjective and the objective assessments. CU Dent. J. 2014, 37, 171–182. [Google Scholar]
- Bhattarai, K.R.; Kim, H.R.; Chae, H.J. Compliance with Saliva Collection Protocol in Healthy Volunteers: Strategies for Managing Risk and Errors. Int. J. Med. Sci. 2018, 15, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Koji, T.; Morita, O. Oral Dryness Examinations: Use of an Oral Moisture Checking Device and a Modified Cotton Method. Prosthodont. Res. Pract. 2006, 5, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, A.M.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral. Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef]
- Dalodom, S.; Lam-ubol, A.; Jeanmaneechotechai, S.; Takamfoo, L.; Intachai, W.; Duangchada, K.; Hongsachum, B.; Kanjanatiwat, P.; Vacharotayangul, P.; Trachootham, D. Influence of oral moisturizing jelly as a saliva substitute for the relief of xerostomia in elderly patients with hypertension and diabetes mellitus. Geriatr. Nurs. 2016, 37, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Sreebny, L.M.; Yu, A.; Green, A.; Valdini, A. Xerostomia in diabetes mellitus. Diabetes Care 1992, 15, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Shirzaiy, M.; Bagheri, F. Prevalence of Xerostomia and its Related Factors in Patients Referred to Zahedan Dental School in Iran. DCEJ 2016, 2, e7138. [Google Scholar] [CrossRef]
- Borhan Mojabi, K.; Esfahani, M.; Jahani Hashemi, H. Evaluation of unstimulated salivary flow rate and oral symptoms in menopausal women. J. Dent. Med. Tehran Univ. Med. Sci. 2007, 4, 103–106. [Google Scholar]
- Paul, T.; Taylor, T.; Babu, R.S.A. Sodium lauryl sulphate. Br. Dent. J. 2019, 227, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachootham, D.; Satoh-Kuriwada, S.; Lam-ubol, A.; Promkam, C.; Chotechuang, N.; Sasano, T.; Shoji, N. Differences in Taste Perception and Spicy Preference: A Thai–Japanese Cross-cultural Study. Chem. Senses. 2017, 43, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Van Loveren, C. Toothpastes. Monogr. Oral. Sci. 2013, 23, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Lakhsmi, M.S. Market Survey of Artificial Sweeteners and Artificially Sweetened Products in Hyderabad. Int. J. Pharm. Biol. Sci. 2019, 9, 37–49. [Google Scholar]
- Folke, S.; Paulsson, G.; Fridlund, B.; Söderfeldt, B. The subjective meaning of xerostomia—An aggravating misery. QHW 2009, 4, 245–255. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; de Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 1, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khovidhunkit, S.O.; Suwantuntula, T.; Thaweboon, S.; Mitrirattanakul, S.; Chomkhakhai, U.; Khovidhunkit, W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: A preliminary study. J. Med. Assoc. Thai. 2009, 92, 1220–1228. [Google Scholar] [PubMed]
- Borahan, M.O.; Pekiner, F.N.; Atalay, T. Evaluation of Effects of the Psychological Factors on Saliva. MUSBED 2012, 2, 8–14. [Google Scholar]
- Gholami, N.; Hosseini Sabzvari, B.; Razzaghi, A.; Salah, S. Effect of stress, anxiety and depression on unstimulated salivary flow rate and xerostomia. J. Dent. Res. Dent. Clin. Dent. Prospects. 2017, 11, 247–252. [Google Scholar] [CrossRef]
- Nadig, S.D.; Ashwathappa, D.T.; Manjunath, M.; Krishna, S.; Annaji, A.G.; Shivaprakash, P.K. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J. Oral. Maxillofac. Pathol. 2017, 21, 316. [Google Scholar] [CrossRef] [Green Version]
- Memtsa, P.T.; Tolia, M.; Tzitzikas, I.; Bizakis, J.; Pistevou-Gombaki, K.; Charalambidou, M.; Iliopoulou, C.; Kyrgias, G. Assessment of xerostomia and its impact on quality of life in head and neck cancer patients undergoing radiation therapy. Mol. Clin. Oncol. 2017, 6, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, A.; Mirzapour, A.; Bijani, A.; Shirzad, A. Salivary flow rate and xerostomia in patients with type I and II diabetes mellitus. Electron. Physician. 2017, 9, 5244–5249. [Google Scholar] [CrossRef] [Green Version]
- Xiang, R.L.; Huang, Y.; Zhang, Y.; Cong, X.; Zhang, Z.J.; Wu, L.L.; Yu, G.Y. Type 2 diabetes-induced hyposalivation of the submandibular gland through PINK1/Parkin-mediated mitophagy. J. Cell Physiol. 2020, 235, 232–244. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.S. The relationship of oral hypoglycemic medications and salivary gland function in type 2 diabetes mellitus patients. Dent. J. 2015, 61, 4175–4184. [Google Scholar]
- Saleh, J.; Figueiredo, M.A.Z.; Cherubini, K.; Salum, F.G. Salivary hypofunction: An update on aetiology, diagnosis and therapeutics. Arch. Oral Biol. 2014, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Fouani, M.; Basset, C.A.; Jurjus, A.R.; Leone, L.G.; Tomasello, G.; Leone, A. Salivary gland proteins alterations in the diabetic milieu. J. Mol. Histol. 2021, 52, 893–904. [Google Scholar] [CrossRef]
- Cui, F.; Hu, M.; Li, R.; Li, B.; Huang, D.; Ma, W.; Jia, X.; Lv, Z. Insulin on changes in expressions of aquaporin-1, aquaporin-5, and aquaporin-8 in submandibular salivary glands of rats with Streptozotocin-induced diabetes. Int. J. Clin. Exp. Pathol. 2021, 14, 221–229. [Google Scholar] [PubMed]
- Stewart, C.R.; Obi, N.; Epane, E.C.; Akbari, A.A.; Halpern, L.; Southerland, J.H.; Gangula, P.R. Effects of diabetes on salivary gland protein expression of tetrahydrobiopterin and nitric oxide synthesis and function. J. Periodontol. 2016, 87, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Isola, M.; Solinas, P.; Proto, E.; Cossu, M.; Lantini, M.S. Reduced statherin reactivity of human submandibular gland in diabetes. Oral Dis. 2011, 17, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Andrades, K.M.R.; Oliveira, G.B.; Ávila, L.F.C.; Odebrecht, M.R.; Miguel, L.C.M. Association of Glycemic indexes, hyposalivation, and xerostomia type 1 diabetic patients. Int. J. Odontostomat. 2011, 5, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Jindal, R.; Shenoy, N.; Denny, C.; Udayalakshmi, J.; Baliga, S. Correlation between Xerostomia, Hyposalivation, and Oral Microbial Load with Glycemic Control in Type 2 Diabetic Patients. World J. Dent. 2016, 7, 83–86. [Google Scholar] [CrossRef]
- De Morais, E.F.; Macedo, R.A.d.P.; Lira, J.A.d.S.; de Lima, K.C.; Borges, B.C.D. Factors related to dry mouth and low salivary flow rates in diabetic elderly: A systematic literature review. Rev. Bras. Geriatr. Gerontol. 2014, 17, 417–423. [Google Scholar] [CrossRef]
- Shetty, S.R.; Bhowmick, S.; Castelino, R.; Babu, S. Drug induced xerostomia in elderly individuals: An institutional study. Contemp. Clin. Dent. 2012, 3, 173–175. [Google Scholar] [CrossRef]
- Petrušić, N.; Posavac, M.; Sabol, I.; Mravak-Stipetić, M. The Effect of Tobacco Smoking on Salivation. Acta Stomatol. Croat. 2015, 49, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, M.G. The diuretic action of alcohol in man. J. Physiol. 1942, 101, 172–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glore, R.J.; Spiteri-Staines, K.; Paleri, V. A patient with dry mouth. Clin. Otolaryngol. 2009, 34, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M. Dry mouth and older people. Aust. Dent. J. 2015, 60, 54–63. [Google Scholar] [CrossRef]
- Maiya, V.M.; Vaid, N.; Basu, S.; Vatyam, S.; Hegde, S.; Deshmukh, S.; Zade, B. The Use of Xylitol for the Prevention of Xerostomia in Patients Receiving Intensity Modulated Radiation Therapy for Head and Neck Cancers. IJROBP 2014, 90, S562. [Google Scholar] [CrossRef]
- Ship, J.A.; McCutcheon, J.A.; Spivakovsky, S.; Kerr, A.R. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J. Oral Rehabil. 2007, 34, 724–732. [Google Scholar] [CrossRef]
- Martín, M.; Marín, A.; López, M.; Liñán, O.; Alvarenga, F.; Büchser, D.; Cerezo, L. Products based on olive oil, betaine, and xylitol in the post-radiotherapy xerostomia. Rep. Pract. Oncol. Radiother. 2017, 22, 71–76. [Google Scholar] [CrossRef] [Green Version]

| Subjective Evaluation of Xerostomia n = Number of Participants | Yes | No |
|---|---|---|
| n (%) | n (%) | |
| 1. Do you feel you have too little saliva in your mouth? | 210 (33.7) | 413 (66.3) |
| 2. Do you have a dry mouth when you eat meals? | 63 (10.1) | 560 (89.9) |
| 3. Do you often have a dry mouth at night or when you wake up in the morning? | 172 (27.6) | 451 (72.4) |
| 4. Do you feel that swallowing your food is difficult? | 58 (9.3) | 565 (90.7) |
| 5. Do you sip water all the time while swallowing food? | 30 (4.8) | 593 (95.2) |
| Total (623) | 239 (38.4) | 384 (61.6) |
| Subjective symptom scores and objective diagnostic tests (n = 104 cases from 239 xerostomia patients) | Mean | SD |
| Xerostomia screening | 2.27 | 1.09 |
| Salivary flow rate measurement (mL/min) | 0.085 | 0.012 |
| Oral moisture (surface of the tongue) (g) | 0.01 | 0.005 |
| Oral moisture (hypoglossus) (g) | 0.09 | 0.03 |
| Variables | Categories | n (%) | No. of Xerostomia Cases (%) | p-Value |
|---|---|---|---|---|
| Sex | Male | 288 (46.2) | 94 (32.6) | 0.006 * |
| Female | 335 (53.8) | 145 (43.3) | ||
| Age (years) | 50–59 | 143 (23.0) | 39 (27.3) | 0.002 * |
| 60–69 | 320 (51.3) | 141 (44.1) | ||
| Over 69 | 160 (25.7) | 59 (36.8) | ||
| Mean = 65.48, SD = 7.73, min. = 50, max. = 88 | ||||
| Toothpaste (type 1) | SLS-free | 68 (11.0) | 26 (38.2) | 0.982 |
| Containing SLS | 555 (89.0) | 213 (38.3) | ||
| Toothpaste (type 2) | Spicy herbal extracts-free | 172 (27.6) | 61 (35.4) | 0.358 |
| Containing spicy herbal extracts | 451 (72.4) | 178 (39.4) | ||
| Toothpaste (type 3) | Artificial sweeteners-free | 368 (59.0) | 146 (39.6) | 0.419 |
| Containing artificial sweeteners | 255 (41.0) | 93 (36.4) | ||
| Education | None | 12 (1.9) | 4 (33.3) | 0.468 |
| Elementary school | 148 (23.8) | 52 (35.1) | ||
| High school | 282 (45.3) | 115 (40.7) | ||
| Bachelor’s degree | 162 (26.0) | 58 (35.8) | ||
| Higher than bachelor’s degree | 19 (3.0) | 10 (52.6) | ||
| Years since diabetes diagnosis | 0–5 | 147 (23.6) | 24 (16.3) | <0.001 ** |
| 6–10 | 241 (38.7) | 76 (31.5) | ||
| Over 10 | 235 (37.7) | 139 (59.1) | ||
| Mean = 10.13, SD = 5.57, min. = 0.10, max. = 32.00 | ||||
| HbA1c (%) | ≤6.5 | 134 (21.5) | 23 (59.1) | <0.001 ** |
| 6.6–6.9 | 126 (20.2) | 32 (25.3) | ||
| ≥7 | 363 (58.3) | 184 (50.6) | ||
| Mean = 8.38, SD = 1.97, min. = 5.70, max. = 13.20 | ||||
| Systemic diseases other than diabetes | None | 135 (21.7) | 16 (11.8) | <0.001 ** |
| Hypertension | 411 (66.0) | 206 (50.1) | <0.001 ** | |
| Dyslipidemia | 381 (61.2) | 191 (50.1) | <0.001 ** | |
| Cardiovascular disorders | 9 (1.4) | 8 (88.8) | 0.002 * | |
| Thyroid disorders | 21 (3.4) | 13 (61.9) | 0.024 * | |
| Hematologic disorders | 11 (1.8) | 8 (72.7) | 0.018 * | |
| Renal disorders | 67 (10.8) | 40 (59.7) | <0.001 ** | |
| Respiratory disorders | 55 (8.8) | 25 (45.4) | 0.257 | |
| Allergy | 109 (17.5) | 47 (43.1) | 0.261 | |
| Gout | 42 (6.7) | 28 (66.6) | <0.001 ** | |
| Medications | None | 137 (22.0) | 22 (16.0) | <0.001 ** |
| Antihypertensive medication | 404 (64.8) | 206 (60.0) | <0.001 ** | |
| Antidyslipidemic agents | 359 (57.6) | 186 (51.8) | <0.001 ** | |
| Antiplatlet and anticoagulant medication | 346 (55.5) | 163 (47.1) | <0.001 ** | |
| Pain medication | 199 (31.9) | 77 (38.6) | 0.907 | |
| Gastrointestinal agents | 16 (2.6) | 7 (43.7) | 0.653 | |
| Cardiovascular medication | 11 (1.8) | 7 (63.6) | 0.082 | |
| Antihistamine | 79 (12.7) | 30 (37.9) | 0.939 | |
| Smoking (cigarettes per day) | Never | 602 (96.6) | 220 (36.5) | <0.001 ** |
| 1–5 | 21(3.4) | 19 (90.4) | ||
| Mean = 0.09, SD = 0.54, min. = 0, max. = 5 | ||||
| Alcohol consumption frequency | Never | 535 (85.9) | 172 (32.1) | <0.001 ** |
| Monthly or less | 41 (6.6) | 31 (75.6) | ||
| 2–4 times a month | 31 (5.0) | 22 (70.9) | ||
| 2–3 times a week | 11 (1.7) | 10 (90.9) | ||
| 4 or more times a week | 5 (0.8) | 4 (80.0) | ||
| Denture wearing | None | 581 (93.3) | 210 (36.1) | <0.001 ** |
| Complete dentures | 6 (0.9) | 6 (100) | ||
| Removable partial dentures | 23 (3.7) | 20 (86.9) | ||
| Fixed partial dentures | 13 (2.1) | 3 (23.0) | ||
| Independent Factors | Number of Cases with Oral Function Problems | Speaking Problem | Tasting Problem | Swallowing Problem | Chewing Problem |
| No problems (%) | 601 (96.5) | 457 (73.4) | 253 (40.6) | 619 (99.4) | |
| Have problems (%) | 22 (3.5) | 166 (26.6) | 370 (59.4) | 4 (0.6) | |
| Categories | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Xerostomia | No (reference) | 3.31 * (1.11–9.80) | 5.12 ** (3.26–8.06) | 3.59 ** (2.32–5.53) | 3.34 * (1.15–5.82) |
| Yes | |||||
| Sex | Male (reference) | 1.29 (0.45–3.69) | 0.79 (0.52–1.20) | 0.99 (0.65–1.51) | 0.98 (0.35–2.74) |
| Female | |||||
| Age (years) | 50–59 (reference) | - | - | - | - |
| 60–69 | 1.69 (0.27–10.27) | 0.81 (0.45–1.45) | 0.95 (0.52–1.73) | 1.50 (0.33–6.84) | |
| Over 69 | 2.54 (0.55–11.77) | 0.71 (0.43–1.16) | 1.00 (0.60–1.64) | 1.34 (0.35–5.12) | |
| Years since diabetes diagnosis | 0–5 (reference) | - | - | - | - |
| 6–10 | 0.81 (0.17–3.67) | 2.00 * (1.05–3.81) | 1.69 (0.93–3.04) | 1.84 (0.19–7.85) | |
| Over 10 | 1.69 (0.44–6.48) | 2.69 * (1.43–5.07) | 1.65 (0.91–2.98) | 7.17 (0.91–8.12) | |
| Denture wearing | No (reference) | 0.98 (0.12–7.69) | 1.77 (0.86–3.64) | 1.54 (0.73–3.23) | 5.45 * (1.65–7.93) |
| Yes | |||||
| Type of denture | None (reference) | - | - | - | - |
| Complete dentures | 8.10 * (2.88–13.95) | 4.99 (0.99–25.09) | 2.00 (1.50–5.22) | 25.90 ** (4.28–56.61) | |
| Removable partial dentures | 2.00 (0.30–4.52) | 2.18 (0.87–5.44) | 2.62 * (1.08–6.37) | 2.35 (0.29–19.06) | |
| Fixed partial dentures | 1.00 (0.42–8.23) | 0.41 (0.05–3.23) | 0.89 (0.19–4.10) | 4.31 (0.51–36.17) |
| Independent Factors | Categories (Total n = 623) | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Sex | Male (reference) | 1.57 | 1.13–2.18 | 0.007 * |
| Female | ||||
| Age (years) | 50–59 (reference) | - | - | - |
| 60–69 | 2.10 | 1.36–3.22 | 0.001 * | |
| Over 69 | 2.55 | 1.95–3.53 | 0.005 * | |
| Toothpaste (type 1) | SLS-free (reference) | 1.00 | 0.59–1.68 | 0.082 |
| Containing SLS | ||||
| Toothpaste (type 2) | Spicy herbal extracts-free (ref.) | 1.86 | 0.82–1.90 | 0.039 * |
| Containing spicy herbal extracts | ||||
| Toothpaste (type 3) | Artificial sweeteners-free (ref.) | 0.87 | 0.62–1.21 | 0.041 * |
| Containing artificial sweeteners | ||||
| Education | None (reference) | - | - | - |
| Elementary school | 0.45 | 0.10–2.01 | 0.297 | |
| High School | 0.48 | 0.18–1.27 | 0.143 | |
| Bachelor’s degree | 0.62 | 0.24–1.57 | 0.314 | |
| Higher than bachelor’s degree | 0.50 | 0.19–1.30 | 0.158 | |
| Years since diabetes diagnosis | 0–5 (reference) | - | - | - |
| 6–10 | 2.36 | 1.41–3.95 | 0.001 * | |
| Over 10 | 7.42 | 4.46–12.34 | <0.001 ** | |
| HbA1c (%) | ≤6.5 (reference) | - | - | - |
| 6.6–6.9 | 1.64 | 0.90–3.00 | 0.106 | |
| ≥7 | 4.96 | 3.02–8.13 | <0.001 ** | |
| Having systemic diseases other than diabetes | No (reference) | 6.25 | 3.60–10.86 | <0.001 ** |
| Yes | ||||
| Systemic diseases other than diabetes | None (reference) | - | - | - |
| Hypertension | 5.45 | 3.58–8.28 | <0.001 ** | |
| Dyslipidemia | 4.06 | 2.79–5.90 | <0.001 ** | |
| Cardiovascular disorders | 13.26 | 1.64–26.73 | 0.015 * | |
| Thyroid disorders | 2.70 | 1.10–6.62 | 0.030* | |
| Hematologic disorders | 4.39 | 1.15–16.74 | 0.030 * | |
| Renal disorders | 2.65 | 1.58–4.46 | <0.001 ** | |
| Respiratory disorders | 1.37 | 0.79–2.40 | 0.259 | |
| Allergy | 1.27 | 0.83–1.93 | 0.262 | |
| Gout | 3.50 | 1.80–6.80 | <0.001 ** | |
| Having medications | No (reference) | 4.21 | 2.58–6.88 | <0.001 ** |
| Yes | ||||
| Medications | None (reference) | - | - | - |
| Antihypertensive medication | 5.86 | 3.85–8.91 | <0.001 ** | |
| Antidyslipidemic agents | 4.28 | 2.97–6.16 | <0.001 ** | |
| Antiplatlet and anticoagulant medication | 2.35 | 1.68–3.30 | <0.001 ** | |
| Pain medication | 1.02 | 0.72–1.44 | 0.907 | |
| Gastrointestinal agents | 1.25 | 0.46–3.42 | 0.654 | |
| Cardiovascular medication | 2.86 | 0.83–9.89 | 0.096 | |
| Antihistamine | 0.98 | 0.60–1.59 | 0.939 | |
| Smoking (cigarettes per day) | Never (reference) | 16.49 | 13.80–17.48 | <0.001 ** |
| 1–5 | ||||
| Alcohol consumption | No (reference) | 6.73 | 3.99–11.35 | <0.001 ** |
| Yes | ||||
| Alcohol consumption frequency | Never (reference) | - | - | - |
| Monthly or less | 6.54 | 3.13–13.65 | <0.001 ** | |
| 2–4 times a month | 5.15 | 2.32–11.44 | <0.001 ** | |
| 2–3 times a week | 21.10 | 2.68–26.18 | 0.004 * | |
| 4 or more times a week | 8.44 | 0.93–16.09 | 0.057 | |
| Denture wearing | No (reference) | 3.94 | 2.00–7.74 | <0.001 ** |
| Yes | ||||
| Type of denture | None (reference) | - | - | - |
| Complete dentures | 7.88 | 2.01–8.93 | 0.039 * | |
| Removable partial dentures | 9.41 | 6.01–12.01 | 0.009 * | |
| Fixed partial dentures | 2.22 | 1.78–6.62 | 0.001 * | |
| Types of toothpastes | Subcategories | OR | 95% CI | p-Value |
| Toothpaste containing SLS (type 1: n = 555) | HbA1c ≤ 6.5% (n = 122) (reference) | 0.24 | 0.14–1.40 | 0.071 |
| HbA1c ≥ 6.6% (n = 433) | ||||
| Toothpaste containing spicy herbal extracts (type 2: n = 451) | HbA1c ≤ 6.5% (n = 97) (reference) | 4.34 | 1.94–5.76 | <0.001 ** |
| HbA1c ≥ 6.6% (n = 354) | ||||
| Toothpaste containing artificial sweeteners (type 3: n = 255) | HbA1c ≤ 6.5% (n = 55) (reference) | 0.36 | 0.17–0.73 | 0.005 * |
| HbA1c ≥ 6.6% (n = 200) |
| Independent Factors | Categories | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Sex | Male (reference) | 1.36 | 1.21–2.61 | <0.001 ** |
| Female | ||||
| Age (years) | 50–59 (reference) | - | - | - |
| 60–69 | 1.51 | 1.29–1.89 | 0.019 * | |
| Over 69 | 2.31 | 1.84–2.53 | 0.026 * | |
| Toothpaste (type 1) | SLS-free (reference) | 1.40 | 0.13–1.67 | 0.096 |
| Containing SLS | ||||
| Toothpaste (type 2) | Spicy herbal extracts-free (ref.) | 9.32 | 3.46–15.25 | 0.032 * |
| Containing spicy herbal extracts | ||||
| Toothpaste (type 3) | Artificial sweeteners-free (ref.) | 0.35 | 0.02–4.82 | 0.013 * |
| Containing artificial sweeteners | ||||
| Years since diabetes diagnosis | 0–5 (reference) | - | - | - |
| 6–10 | 1.08 | 0.03–1.18 | <0.001 ** | |
| Over 10 | 2.40 | 0.23–4.68 | 0.001 * | |
| HbA1c (%) | ≤ 6.5 (reference) | - | - | - |
| 6.6–6.9 | 5.10 | 1.05–9.21 | <0.001 ** | |
| ≥ 7 | 8.17 | 2.08–12.34 | <0.001 ** | |
| Type of denture | None (reference) | - | - | - |
| Complete dentures | 3.66 | 1.51–5.96 | 0.003 * | |
| Removable partial dentures | 8.59 | 1.60–12.55 | 0.001 * | |
| Fixed partial dentures | 1.97 | 1.00–19.95 | 0.004 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonpanao, P.; Janebodin, K.; Namvichaisirikul, N.; Thongjit, S.; Jitprasertwong, P. The Prevalence of Xerostomia in Older Thai Individuals with Type II Diabetes Mellitus and Its Association with Type of Toothpaste and Oral Functions: A Cross-Sectional Study Using Questionnaires. Geriatrics 2023, 8, 76. https://doi.org/10.3390/geriatrics8040076
Sonpanao P, Janebodin K, Namvichaisirikul N, Thongjit S, Jitprasertwong P. The Prevalence of Xerostomia in Older Thai Individuals with Type II Diabetes Mellitus and Its Association with Type of Toothpaste and Oral Functions: A Cross-Sectional Study Using Questionnaires. Geriatrics. 2023; 8(4):76. https://doi.org/10.3390/geriatrics8040076
Chicago/Turabian StyleSonpanao, Panitan, Kajohnkiart Janebodin, Niwatchai Namvichaisirikul, Supattarayan Thongjit, and Paiboon Jitprasertwong. 2023. "The Prevalence of Xerostomia in Older Thai Individuals with Type II Diabetes Mellitus and Its Association with Type of Toothpaste and Oral Functions: A Cross-Sectional Study Using Questionnaires" Geriatrics 8, no. 4: 76. https://doi.org/10.3390/geriatrics8040076
APA StyleSonpanao, P., Janebodin, K., Namvichaisirikul, N., Thongjit, S., & Jitprasertwong, P. (2023). The Prevalence of Xerostomia in Older Thai Individuals with Type II Diabetes Mellitus and Its Association with Type of Toothpaste and Oral Functions: A Cross-Sectional Study Using Questionnaires. Geriatrics, 8(4), 76. https://doi.org/10.3390/geriatrics8040076