Interplay between Fungal Infection and Bacterial Associates in the Wax Moth Galleria mellonella under Different Temperature Conditions
Abstract
:1. Introduction
2. Material and Methods
2.1. Fungi and Insects
2.2. Procedures for Infection and Bioassays
2.3. Bacterial Colony Forming Unit (CFU) Counts
2.4. Analysis of Bacterial Communities
2.5. Gene Expression
2.6. In Vitro Interaction between Fungi and Bacteria
2.7. Statistics
3. Results
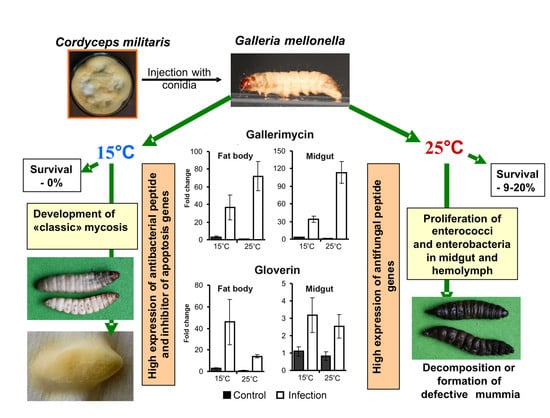
3.1. Bioassays
3.2. CFU Counts in the Hemolymph and Midgut
3.3. Bacterial Communities in Midguts and Cadavers
3.4. AMP Gene Expression
3.5. Apoptosis, ROS and Stress-Related Gene Expression
3.6. Interaction between Fungi and Bacteria In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vey, A.; Fargues, J. Histological and ultrastructural studies of Beauveria bassiana infection in Leptinotarsa decemlineata larvae during ecdysis. J. Invertebr. Pathol. 1977, 30, 207–215. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Fungus interacts with gut bacteria to kill insect. PNAS 2017, 201703546. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.L.; Muturi, E.J.; Dunlap, C.; Rooney, A.P. Strain-specific pathogenicity and subversion of phenoloxidase activity in the mosquito Aedes aegypti by members of the fungal entomopathogenic genus Isaria. Sci. Rep. 2018, 8, 9896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, X.X.; Zhang, X.C.; Zhang, S.; Lu, J.; Xia, Y.M.; Huang, Y.H.; Wang, X.J. The interactions between gut microbiota and entomopathogenic fungi: A potential approach for biological control of Blattella germanica (L.). Pest Manag. Sci. 2018, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Deng, J.; Zhou, F.; Cheng, C.; Zhang, L.; Zhang, J.; Lu, M. Gut microbiota in an invasive bark beetle infected by a pathogenic fungus accelerates beetle mortality. J. Pest Sci. 2018, 92, 343–351. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, X.; Xu, L.; Guo, S.; Chen, G.; Zhang, X. Repressed Beauveria bassiana infections in Delia antiqua due to associated microbiota. Pest Manag. Sci. 2019, 75, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Polenogova, O.V.; Kabilov, M.R.; Tyurin, M.V.; Rotskaya, U.N.; Krivopalov, A.V.; Morozova, V.V.; Mozhaitseva, K.; Kryukova, N.A.; Alikina, T.; Kryukov, V.Y.; et al. Parasitoid envenomation alters the Galleria mellonella midgut microbiota and immunity, thereby promoting fungal infection. Sci. Rep. 2019, 9, 4012. [Google Scholar] [CrossRef] [Green Version]
- Noskov, Y.A.; Kabilov, M.R.; Polenogova, O.V.; Yurchenko, Y.A.; Belevich, O.E.; Yaroslavtseva, O.N.; Alikina, T.Y.; Byvaltsev, A.M.; Rotskaya, U.N.; Morozova, V.V.; et al. A neurotoxic insecticide promotes fungal infection in Aedes aegypti larvae by altering the bacterial community. Microb. Ecol. 2020. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Menzies, F.V.; Kamp, A.M. Genetic groups of the insect pathogenic fungus Beauveria bassiana are associated with habitat and thermal growth preferences. Arch. Microbiol. 2002, 178, 531–537. [Google Scholar] [CrossRef]
- Ouedraogo, R.M.; Cusson, M.; Goettel, M.S.; Brodeur, J. Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var acridum. J. Invertebr. Pathol. 2003, 82, 103–109. [Google Scholar] [CrossRef]
- Ouedraogo, R.M.; Goettel, M.S.; Brodeur, J. Behavioral thermoregulation in the migratory locust: A therapy to overcome fungal infection. Oecologia 2004, 138, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; James, R.R. Temperature stress affects the expression of immune response genes in the alfalfa leafcutting bee, Megachile rotundata: Bee immunity gene expression and temperature. Insect Mol. Biol. 2012, 21, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Keyser, C.A.; Fernandes, É.K.K.; Rangel, D.E.N.; Roberts, D.W. Heat-induced post-stress growth delay: A biological trait of many Metarhizium isolates reducing biocontrol efficacy? J. Invertebr. Pathol. 2014, 120, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Hunt, V.L.; Zhong, W.; McClure, C.D.; Mlynski, D.T.; Duxbury, E.M.L.; Keith Charnley, A.; Priest, N.K. Cold-seeking behaviour mitigates reproductive losses from fungal infection in Drosophila. J. Anim. Ecol. 2016, 85, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, C.; Fargues, J. Climatic Constraints for Fungal Biopesticides. In Use of Entomopathogenic Fungi in Biological Pest Management; Ekesi, S., Maniana, N.K., Eds.; Res. Signpost: Kerala, India, 2007; pp. 39–55. [Google Scholar]
- Linder, J.E.; Owers, K.A.; Promislow, D.E.L. The effects of temperature on host–pathogen interactions in D. melanogaster: Who benefits? J. Insect Physiol. 2008, 54, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, L.M.; Tisa, L.S. Influence of Temperature on the Physiology and Virulence of the Insect Pathogen Serratia sp. Strain SCBI. Appl. Environ. Microbiol. 2012, 78, 8840–8844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mowlds, P.; Kavanagh, K. Effect of pre-incubation temperature on susceptibility of Galleria mellonella larvae to infection by Candida albicans. Mycopathologia 2008, 165, 5–12. [Google Scholar] [CrossRef]
- Zibaee, A.; Bandani, A.R.; Talaei-Hassanlouei, R.; Malagoli, D. Temperature and Ca2+ ion as modulators in cellular immunity of the Sunn pest Eurygaster integriceps Puton (Heteroptera: Scutelleridae). Entomol. Res. 2009, 39, 364–371. [Google Scholar] [CrossRef]
- Fuller, C.A.; Postava-Davignon, M.A.; West, A.; Rosengaus, R.B. Environmental conditions and their impact on immunocompetence and pathogen susceptibility of the Caribbean termite Nasutitermes acajutlae. Ecol. Entomol. 2011, 36, 459–470. [Google Scholar] [CrossRef]
- Catalán, T.P.; Wozniak, A.; Niemeyer, H.M.; Kalergis, A.M.; Bozinovic, F. Interplay between thermal and immune ecology: Effect of environmental temperature on insect immune response and energetic costs after an immune challenge. J. Insect Physiol. 2012, 58, 310–317. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Tomilova, O.G.; Yaroslavtseva, O.N.; Wen, T.C.; Kryukova, N.A.; Polenogova, O.V.; Tokarev, Y.S.; Glupov, V.V. Temperature adaptations of Cordyceps militaris, impact of host thermal biology and immunity on mycosis development. Fungal Ecol. 2018, 35, 98–107. [Google Scholar] [CrossRef]
- Kryukov, V.; Yaroslavtseva, O.N.; Whitten, M.M.A.; Tyurin, M.V.; Ficken, K.; Greig, C.; Melo, N.R.; Glupov, V.V.; Dubovskiy, I.M.; Butt, T. Fungal infection dynamics in response to temperature in the lepidopteran insect Galleria mellonella. Insect Sci. 2018, 25, 454–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamakhi, L.; Zibaee, A.; Karimi-Malati, A.; Hoda, H. Effect of thermal stress on the immune responses of Chilo suppressalis Walker (Lepidoptera: Crambidae) to Beauveria bassiana. J. Therm. Biol. 2019, 84, 136–145. [Google Scholar] [CrossRef]
- Adamo, S.A.; Lovett, M.M. Some like it hot: The effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. J. Exp. Biol. 2011, 214, 1997–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehipour-shirazi, G.; Ferguson, L.V.; Sinclair, B.J. Does cold activate the Drosophila melanogaster immune system? J. Insect Physiol. 2017, 96, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.V.; Sinclair, B.J. Insect immunity varies idiosyncratically during overwintering. J. Exp. Zool. 2017, 327, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Wojda, I.; Taszłow, P.; & Jakubowicz, T. The effect of cold shock on the immune response of the greater wax moth Galleria mellonella after infection with entomopathogenic bacteria Bacillus thuringiensis. Annales UMCS Sec. C 2014, 69, 7–18. [Google Scholar] [CrossRef]
- Chen, K.; Tang, T.; Song, Q.; Wang, Z.; He, K.; Liu, X.; Song, J.; Wang, L.; Yang, Y.; Feng, C. Transcription Analysis of the Stress and Immune Response Genes to Temperature Stress in Ostrinia furnacalis. Front. Physiol. 2019, 10, 1289. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Miyado, K.; Takezawa, Y.; Ohnami, N.; Sato, M.; Ono, C.; Harada, Y.; Yoshida, K.; Kawano, N.; Kanai, S.; et al. Innate immune system still works at diapause, a physiological state of 453 dormancy in insects. Biochem. Biophys. Res. Comm. 2011, 410, 351–357. [Google Scholar] [CrossRef]
- Ferguson, L.V.; Dhakal, P.; Lebenzon, J.E.; Heinrichs, D.E.; Bucking, C.; Sinclair, B.J. Seasonal shifts in the insect gut microbiome are concurrent with changes in cold tolerance and immunity. Funct. Ecol. 2018, 32, 2357–2368. [Google Scholar] [CrossRef]
- Balandin, S.V.; Ovchinnikova, T.V. Antimicrobial peptides of invertebrates. Part 1. Structure, biosynthesis, and evolution. Russ. J. Bioorganic Chem. 2016, 42, 229–248. [Google Scholar] [CrossRef]
- Schuhmann, B.; Seitz, V.; Vilcinskas, A.; Podsiadlowski, L. Cloning and expression of gallerimycin, an antifungal peptide expressed in immune response of greater wax moth larvae, Galleria mellonella. Arch. Insect Biochem. Physiol. 2003, 53, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yun, E.K.; Jang, W.S.; Kim, I.; Lee, J.H.; Park, S.Y.; Ryu, K.S.; Seo, S.J.; Kim, C.H.; Lee, I.H. Purfication, cDNA cloning and expression of an insect defensin from the great wax moth, Galleria mellonella. Insect Mol. Biol. 2004, 13, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017, 24, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Vilcinskas, A. The entomopathogenic fungus Metarhizium robertsii communicates with the insect host Galleria mellonella during infection. Virulence 2018, 9, 402–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Keyhani, N.O.; Zhang, H.; Cai, K.; Xia, Y. Inhibitor of apoptosis-1 gene as a potential target for pest control and its involvement in immune regulation during fungal infection. Pest. Manag. Sci. 2020, 76, 1831–1840. [Google Scholar] [CrossRef]
- Leulier, F.; Lhocine, N.; Lemaitre, B.; Meier, P. The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol. Cell. Biol. 2006, 26, 7821–7831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef]
- Ha, E.M.; Oh, C.T.; Bae, Y.S.; Lee, W.J. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005, 310, 847–850. [Google Scholar] [CrossRef]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, L.; Pang, X.; Zhang, R.; Zhu, Y.; Wang, P.; Gao, G.; Cheng, G. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nat. Microbiol. 2017, 2, 17020. [Google Scholar] [CrossRef] [PubMed]
- Slepneva, I.A.; Glupov, V.V.; Sergeeva, S.V.; Khramtsov, V.V. EPR detection of reactive oxygen species in hemolymph of Galleria mellonella and Dedrolimus superans sibiricus (Lepidoptera) larvae. Biochem. Biophis. Res. Com. 1999, 264, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Slepneva, I.A.; Komarov, D.A.; Glupov, V.V.; Serebrov, V.V.; Khramtsova, V.V. Infuence of fungal in-fection on the DOPA-semiquinone and DOPA-quinone production in haemolymph of Galleria mellonella larvae. Biochem. Biophis. Res. Com. 2003, 300, 188–191. [Google Scholar] [CrossRef]
- Komarov, D.A.; Slepneva, I.A.; Glupov, V.V.; Khramtsov, V.V. Superoxide and hydrogen peroxide formation during enzymatic oxidation of DOPA by phenoloxidase. Free Radic. Res. 2005, 39, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jones, W.A. Expression of heat shock protein genes in insect stress responses. ISJ 2012, 9, 93–101. [Google Scholar]
- Cheng, W.N.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K.Y. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, X.; Keyhani, N.O.; Tang, G.; Pei, Y.; Zhang, W.; Tong, S. Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proc. Natl. Acad. Sci. USA 2017, 114, E1578–E1586. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Li, M.; Keyhani, N.O.; Liu, Y.; Yuan, M.; Lin, D.; Jin, D.; Li, X.; Pei, Y.; Fan, Y. Characterization of a fungal competition factor: Production of a conidial cell-wall associated antifungal peptide. PLoS Pathog. 2020, 16, e1008518. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Xia, Y.L.; Xiao, G.H.; Xiong, C.H.; Hu, X.; Zhang, S.W.; Zheng, H.J.; Huang, Y.; Zhou, Y.; Wang, S.Y.; et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol. 2011, 12, R116. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Xiao, G.; Zheng, P.; Shang, Y.; Su, Y.; Zhang, X.; Liu, X.; Zhan, S.; Leger, R.J.; Wang, C. Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proc. Natl. Acad. Sci. USA 2014, 111, 16796–16801. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-J.; Luo, F.; Li, B.; Shang, Y.; Wang, C. Metabolic conservation and diversification of Metarhizium species correlate with fungal host-specificityFront. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Shang, Y.; St. Leger, R.J.; Zhao, G.P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamata, N. Population dynamics of the beech caterpillar, Syntypistis punctatella, and biotic and abiotic factors. Popul. Ecol. 2000, 42, 267–278. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Lednev, G.R.; Borisov, B.A. Local epizootics caused by teleomorphic cordycipitoid fungi (Ascomycota: Hypocreales) in populations of forest lepidopterans and sawflies of the summer-autumn complex in Siberia. Microbiology 2011, 80, 286–295. [Google Scholar] [CrossRef]
- Gedminas, A.; Lynikiene, J.; Povilaitiene, A. Entomopathogenic fungus Cordyceps militaris: Distribution in South Lithuania, in vitro cultivation and pathogenicity tests. Balt. For. 2015, 21, 359–368. [Google Scholar]
- Harada, Y.; Akiyama, N.; Yamamoto, K.; Shirota, Y. Production of Cordyceps militaris fruit body on artificially inoculated pupae of Mamestra brassicae in the laboratory. Nippon Kingakukai Kaiho 1995, 36, 63–72. [Google Scholar]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Kukharenko, A.E.; Glupov, V.V. Stromata cultivation of entomopathogenic fungus Cordyceps militaris (Hypocreales) on nonspecific hosts. Mikologiya I Fitopatologiya 2012, 46, 269–272. (In Russian) [Google Scholar]
- Kryukov, V.Y.; Kryukova, N.A.; Tomilova, O.G.; Vorontsova, Y.; Chertkova, E.; Pervushin, A.L.; Slepneva, I.; Glupov, V.V.; Yaroslavtseva, O.N. Comparative analysis of the immune response of the wax moth Galleria mellonella after infection with the fungi Cordyceps militaris and Metarhizium robertsii. Microb. Pathog. 2020, 141, 103995. [Google Scholar] [CrossRef]
- Basith, M.; Madelin, M.F. Studies on the production of perithecial stromata by Cordyceps militaris in artificial culture. Can. J. Bot. 1968, 46, 473–480. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Whitten, M.M.A.; Kryukov, V.Y.; Yaroslavtseva, O.N.; Grizanova, E.V.; Greig, C.; Mukherjee, K.; Vilcinskas, A.; Mitkovets, P.V.; Glupov, V.V.; et al. More than a color change: Insect melanism, disease resistance and fecundity. Proc. Royal Soc. B 2013, 280, 20130584. [Google Scholar] [CrossRef] [PubMed]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igolkina, A.A.; Grekhov, G.A.; Pershina, E.V.; Samosorov, G.G.; Leunova, V.M.; Semenov, A.N.; Baturina, O.A.; Kabilov, M.R.; Andronova, E.E. Identifying components of mixed and contaminated soil samples by detecting specific signatures of control 16S rRNA libraries. Ecol. Indic. 2018, 94, 446–453. [Google Scholar] [CrossRef]
- Edgar, R. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. SINTAX: A simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv 2016. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Lange, A.; Schäfer, A.; Bender, A.; Steimle, A.; Beier, S.; Parusel, R.; Frick, J.-S. Galleria mellonella: A novel invertebrate model to distinguish intestinal symbionts from pathobionts. Front. Immun. 2018, 19, 2114. [Google Scholar] [CrossRef] [Green Version]
- Melo, N.R.; Abdrahman, A.; Greig, C.; Mukherjee, K.; Thornton, C.; Ratcliffe, N.A.; Vilcinskas, A.; Butt, T.M. Myriocin significantly increases the mortality of a non-mammalian model host during Candida pathogenesis. PLoS ONE 2013, 8, e78905. [Google Scholar] [CrossRef] [Green Version]
- Scheirer, C.J.; Ray, W.S.; Hare, N. The analysis of ranked data derived from completely randomized factorial designs. Biometrics 1976, 32, 429–434. [Google Scholar] [CrossRef]
- Sato, H.; Shimazu, M. Stromata production for Cordyceps militaris (Clavicipitales: Clavicipitaceae) by injection of hyphal bodies to alternative host insects. Appl. Entomol. Zool. 2002, 37, 85–92. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Surina, E.V.; Tyurin, M.V.; Dubovskiy, I.M.; Glupov, V.V. Immune reactions of the greater wax moth, Galleria mellonella L. (lepidoptera, pyralidae) larvae under combined treatment of the entomopathogens Cordyceps militaris (L.: Fr.) Link and Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota, Hypocreales). Entmol. Rev. 2015, 95, 693–698. [Google Scholar] [CrossRef]
- Johnston, P.R.; Rolff, J. Host and symbiont jointly control gut microbiota during complete metamorphosis. PLoS Pathog. 2015, 11, e1005246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allonsius, C.N.; Van Beeck, W.; De Boeck, I.; Wittouck, S.; Lebeer, S. The microbiome of the invertebrate model host Galleria mellonella is dominated by Enterococcus. Anim. Microbiome 2019, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Dubovskiy, I.M.; Grizanova, E.V.; Whitten, M.M.A.; Mukherjee, K.; Greig, C.; Alikina, T.; Kabilov, M.; Vilcinskas, A.; Glupov, V.V.; Butt, T.M. Immuno-physiological adaptations confer wax moth Galleria mellonella resistance to Bacillus thuringiensis. Virulence 2016, 7, 860–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryukov, V.; Kabilov, M.; Tomilova, O.; Yaroslavtseva, O. Metagenomics of Galleria mellonella midgut bacterial community during development of mycoses caused by Cordyceps militaris and Metarhizium robertsii. Unpublished.
- An, Y.; Li, Y.; Wang, X.; Chen, Z.; Xu, H.; Wu, L.; Li, S.; Wang, C.; Luan, W.; Wang, X.; et al. Cordycepin reduces weight through regulating gut microbiota in high-fat diet-induced obese rats. Lipids Health Dis. 2018, 17, 276. [Google Scholar] [CrossRef] [Green Version]
- Gamage, S.; Nakayama, J.; Fuyuno, Y.; Ohga, S. The effect of the hot water extracts of the Paecilomyces hepiali and Cordyceps militaris mycelia on the growth of gastrointestinal bacteria. Adv. Microbiol. 2018, 8, 490–505. [Google Scholar] [CrossRef] [Green Version]
- Kryukov, V.Y.; Rotskaya, U.; Yaroslavtseva, O.; Polenogova, O.; Kryukova, N.; Akhanaev, Y.; Krivopalov, A.; Alikina, T.; Vorontsova, Y.; Slepneva, I.; et al. Fungus Metarhizium robertsii and neurotoxic insecticide affect the gut immunity and microbiota in Colorado potato beetle. Sci. Rep. Under review.
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. Diet influences the bacterial and free fatty acid profiles of the cuticle of Galleria mellonella larvae. PLoS ONE 2019, 14, e0211697. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, W.J. Role of DUOX in gut inflammation: Lessons from Drosophila model of gut-microbiota interactions. Front. Cell. Infect. Microbiol. 2014, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Wrońska, A.K.; Boguś, M.I. Heat shock proteins (HSP 90, 70, 60, and 27) in Galleria mellonella (Lepidoptera) hemolymph are affected by infection with Conidiobolus coronatus (Entomophthorales). PLoS ONE 2020, 15, e0228556. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Leelatanawit, R.; Jiravanichpaisal, P.; Klinbunga, S.; Karoonuthaisiri, N. Expression and distribution of three heat shock protein genes under heat shock stress and under exposure to Vibrio harveyi in Penaeus monodon. Dev. Comp. Immunol. 2010, 34, 1082–1089. [Google Scholar] [CrossRef]
- Wojda, I.; Kowalski, P. Galleria mellonella infected with Bacillus thuringiensis involves Hsp90. Cent. Eur. J. Biol. 2013, 8, 561–569. [Google Scholar] [CrossRef]
- Wojda, I.; Jakubowicz, T. Humoral immune response upon mild heat-shock conditions in Galleria mellonella larvae. J. Insect Physiol. 2007, 53, 1134–1144. [Google Scholar] [CrossRef] [PubMed]








| Effects | |||
|---|---|---|---|
| Infection | Temperature | Infection × Temperature | |
| Fat body | |||
| Galiomycin | ↑H1,19 = 14.3 p < 0.001 | H1,19 = 0.0 p = 1.00 | H1,19 = 0.7 p = 0.40 |
| Gallerimycin | ↑H1,19 = 14.3 p < 0.001 | H1,19 = 0.0 p = 0.88 | H1,19 = 2.1 p = 0.15 |
| Gloverin | ↑H1,19 = 14.3 p < 0.001 | H1,19 = 1.5 p = 0.23 | H1,19 = 0.1 p = 0.82 |
| Cecropin | ↑H1,19 = 3.3 p = 0.08 | ↑H1,19 = 3.9 p = 0.05 | H1,19 = 0.1 p = 0.76 |
| Lysozyme | ↑H1,23 = 12.8 p < 0.001 | ↑H1,23 = 3.2 p = 0.07 | H1,23 = 0.1 p = 0.77 |
| Midgut | |||
| Galiomycin | ↑H1,19 = 10.1 p = 0.002 | H1,19 = 1.2 p = 0.29 | H1,19 = 1.3 p = 0.27 |
| Gallerimycin | ↑H1,19 = 14.3 p < 0.001 | H1,19 = 0.1 p = 0.76 | H1,19 = 2.5 p = 0.11 |
| Gloverin | ↑H1,19 = 6.2 p = 0.01 | H1,19 = 0.2 p = 0.65 | H1,19 = 0.0 p = 0.88 |
| Cecropin | H1,23 = 1.2 p = 0.27 | H1,23 = 1.0 p = 0.33 | H1,23 = 0.5 p = 0.49 |
| Lysozyme | ↑H1,23 = 9.4 p = 0.002 | ↓H1,23 = 4.6 p = 0.03 | H1,23 = 1.1 p = 0.30 |
| Effects | |||
|---|---|---|---|
| Infection | Temperature | Infection × temperature | |
| Fat body | |||
| IAP | H1,23 = 0.8 p = 0.39 | ↑H1,23 = 7.4 p = 0.007 | H1,23 = 3.0 p = 0.08 |
| NOX-DUOX | ↑H1,23 = 4.3 p = 0.04 | H1,23 = 0.6 p = 0.45 | H1,23 = 0.0 p = 0.86 |
| Hsp70 | ↓H1,23 = 4.6 p = 0.03 | ↑H1,23 = 14.5 p < 0.001 | H1,23 = 0.0 p = 0.95 |
| Hsp90 | ↑H1,23 = 2.8 p = 0.09 | H1,23 = 0.3 p = 0.56 | H1,23 = 0.1 p = 0.77 |
| Midgut | |||
| IAP | ↑H1,23 = 7.7 p = 0.006 | H1,23 = 0.0 p = 0.91 | H1,23 = 0.0 p = 0.91 |
| NOX-DUOX | H1,23 = 1.8 p = 0.18 | ↓H1,23 = 6.2 p = 0.01 | H1,23 = 1.3 p = 0.25 |
| Hsp70 | H1,23 = 0.0 p = 0.93 | ↑H1,23 = 13.7 p < 0.001 | H1,23 = 0.1 p = 0.71 |
| Hsp90 | H1,23 = 0.2 p = 0.64 | ↑H1,23 = 8.7 p = 0.003 | H1,23 = 0.8 p = 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryukov, V.Y.; Kosman, E.; Tomilova, O.; Polenogova, O.; Rotskaya, U.; Tyurin, M.; Alikina, T.; Yaroslavtseva, O.; Kabilov, M.; Glupov, V. Interplay between Fungal Infection and Bacterial Associates in the Wax Moth Galleria mellonella under Different Temperature Conditions. J. Fungi 2020, 6, 170. https://doi.org/10.3390/jof6030170
Kryukov VY, Kosman E, Tomilova O, Polenogova O, Rotskaya U, Tyurin M, Alikina T, Yaroslavtseva O, Kabilov M, Glupov V. Interplay between Fungal Infection and Bacterial Associates in the Wax Moth Galleria mellonella under Different Temperature Conditions. Journal of Fungi. 2020; 6(3):170. https://doi.org/10.3390/jof6030170
Chicago/Turabian StyleKryukov, Vadim Yu, Elena Kosman, Oksana Tomilova, Olga Polenogova, Ulyana Rotskaya, Maksim Tyurin, Tatyana Alikina, Olga Yaroslavtseva, Marsel Kabilov, and Viktor Glupov. 2020. "Interplay between Fungal Infection and Bacterial Associates in the Wax Moth Galleria mellonella under Different Temperature Conditions" Journal of Fungi 6, no. 3: 170. https://doi.org/10.3390/jof6030170
APA StyleKryukov, V. Y., Kosman, E., Tomilova, O., Polenogova, O., Rotskaya, U., Tyurin, M., Alikina, T., Yaroslavtseva, O., Kabilov, M., & Glupov, V. (2020). Interplay between Fungal Infection and Bacterial Associates in the Wax Moth Galleria mellonella under Different Temperature Conditions. Journal of Fungi, 6(3), 170. https://doi.org/10.3390/jof6030170