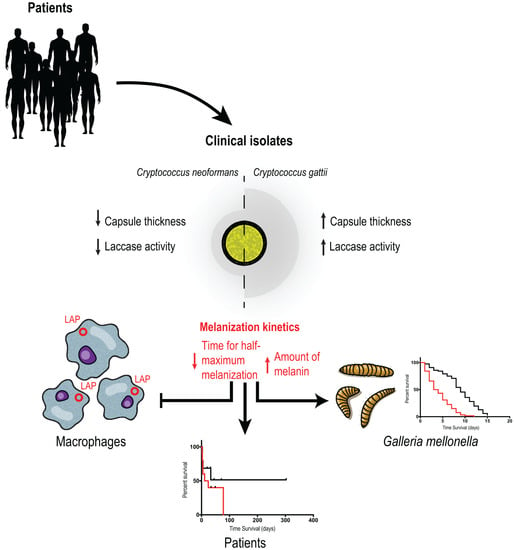
Faster Cryptococcus Melanization Increases Virulence in Experimental and Human Cryptococcosis
Abstract
:1. Introduction
2. Methods
2.1. Patients and Cryptococcus spp. Isolates
2.2. Cryptococcus Culture
2.3. Melanin Production Evaluation
2.4. Semi-Quantitative Melanization Score
2.5. Melanin Quantification in Grayscale
2.6. Laccase Activity
2.7. ELISA for GXM Quantitation Secreted in Supernatant
2.8. Capsule Formation
2.9. Analysis of Extracellular Vesicles (EVs)
2.10. Interaction with Macrophages—Immunofluorescence Microscopy and LC3-Associated Phagocytosis (LAP)
2.11. G. mellonella Median Survival Time
2.12. Statistical Power
2.13. Statistics
3. Results
3.1. Clinical and Epidemiological Data
3.2. Melanization Kinetics
3.3. Clinical Isolates Demonstrate the Variation of Secreted GXM and Capsule Size in Different Culture Media
3.4. Clinical Isolates Present Different Profiles of Interaction with Macrophages
3.5. C. gattii Isolates Have Larger Capsules and More Secreted Laccase Activity, but C. neoformans Melanizes Faster and More Intensely
3.6. Amount of Secreted Extracellular Vesicles Correlates with Capsule Thickness, Melanization and Secreted Laccase Activity
3.7. Melanization Kinetics of Clinical Isolates Affect Ability to Escape from LC3-Associated Phagocytosis in Macrophages
3.8. Melanization Kinetics, Laccase Activity and Capsule of Clinical Isolates Affect Survival in G. mellonella
3.9. Secreted Laccase Activity and tHMM Increases Risk of Death in Patients with Disseminated Cryptococcosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.; Loyse, A.; Boulware, D. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J.; Bennett, J.E.; Wickes, B.L.; Meyer, W.; Cuomo, C.A.; Wollenburg, K.R.; Bicanic, T.A.; Castañeda, E.; Chang, Y.C.; Chen, J.; et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere 2017, 2, e00357-16. [Google Scholar] [CrossRef] [Green Version]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Heitman, J. The Biology of the Cryptococcus neoformans Species Complex. Annu. Rev. Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Kwon-Chung, K.J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 1994, 14, 4912–4919. [Google Scholar] [CrossRef] [PubMed]
- Kozubowski, L.; Lee, S.C.; Heitman, J. Signalling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 2009, 11, 370–380. [Google Scholar] [CrossRef] [Green Version]
- McClelland, E.E.; Bernhardt, P.; Casadevall, A. Estimating the Relative Contributions of Virulence Factors for Pathogenic Microbes. Infect. Immun. 2006, 74, 1500–1504. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza, O. Basic principles of the virulence of Cryptococcus. Virulence 2019, 10, 490–501. [Google Scholar] [CrossRef] [Green Version]
- Denham, S.T.; Verma, S.; Reynolds, R.C.; Worne, C.L.; Daugherty, J.M.; Lane, T.E.; Brown, J.C.S. Regulated Release of Cryptococcal Polysaccharide Drives Virulence and Suppresses Immune Cell Infiltration into the Central Nervous System. Infect. Immun. 2018, 86, e00662-17. [Google Scholar] [CrossRef] [Green Version]
- Robertson, E.J.; Najjuka, G.; Rolfes, M.A.; Akampurira, A.; Jain, N.; Anantharanjit, J.; von Hohenberg, M.; Tassieri, M.; Carlsson, A.; Meya, D.B.; et al. Cryptococcus neoformans Ex Vivo Capsule Size Is Associated with Intracranial Pressure and Host Immune Response in HIV-associated Cryptococcal Meningitis. J. Infect. Dis. 2013, 209, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Casadevall, A. Impact of Melanin on Microbial Virulence and Clinical Resistance to Antimicrobial Compounds. Antimicrob. Agents Chemother. 2006, 50, 3519–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, N.T.; Casadevall, A. Physiological Differences in Cryptococcus neoformans Strains In Vitro versus In Vivo and Their Effects on Antifungal Susceptibility. Antimicrob. Agents Chemother. 2017, 61, e02108-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, P.R.; Wakamatsu, K.; Ito, S. Melanin Biosynthesis in Cryptococcus neoformans. J. Bacteriol. 1998, 180, 1570–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabiiti, W.; Robertson, E.; Beale, M.; Johnston, S.; Brouwer, A.E.; Loyse, A.; Jarvis, J.; Gilbert, A.S.; Fisher, M.; Harrison, T.; et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J. Clin. Investig. 2014, 124, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- Rosas Ángel, L.; Nosanchuk, J.D.; Gómez, B.L.; Edens, W.A.; Henson, J.M.; Casadevall, A. Isolation and serological analyses of fungal melanins. J. Immunol. Methods 2000, 244, 69–80. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; España, J.D.A.; De Alencar, L.P.; Pereira, V.S.; Castelo-Branco, D.; Pereira-Neto, W.D.A.; Cordeiro, R.D.A.; Sidrim, J.J.C.; Rocha, M.F.G. An alternative method for the analysis of melanin production in Cryptococcus neoformans sensu lato and, Cryptococcus gattii sensu lato. Mycoses 2017, 60, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Cunha, M.M.; Franzen, A.J.; Seabra, S.H.; Herbst, M.H.; Vugman, N.V.; Borba, L.P.; de Souza, W.; Rozental, S. Melanin in Fonsecaea pedrosoi: A trap for oxidative radicals. BMC Microbiol. 2010, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Bach, C.E.; Warnock, D.D.; Van Horn, D.J.; Weintraub, M.N.; Sinsabaugh, R.L.; Allison, S.D.; German, D.P. Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA and ABTS: Effect of assay conditions and soil type. Soil Biol. Biochem. 2013, 67, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Sinsabaugh, R.; Saiya-Cork, K.; Long, T.; Osgood, M.; Neher, D.; Zak, D.; Norby, R. Soil microbial activity in a Liquidambar plantation unresponsive to CO2-driven increases in primary production. Appl. Soil Ecol. 2003, 24, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Ost, K.S.; O’meara, T.R.; Huda, N.; Esher, S.K.; Alspaugh, J.A. The Cryptococcus neoformans Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator. PLoS Genet. 2015, 11, e1005159. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza, O.; Casadevall, A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proced. Online 2004, 6, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.L.; Frases, S.; Miranda, K.; Zaragoza, O.; Alvarez, M.; Nakouzi, A.; Feldmesser, M.; Casadevall, A. Vesicular Polysaccharide Export in Cryptococcus neoformans Is a Eukaryotic Solution to the Problem of Fungal Trans-Cell Wall Transport. Eukaryot. Cell 2007, 6, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Canadien, V.; Lam, G.Y.; Steinberg, B.E.; Dinauer, M.C.; Magalhaes, M.A.O.; Glogauer, M.; Grinstein, S.; Brumell, J.H. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.-C.; Kontoyiannis, D.P.; et al. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrmizi, I.; Ferreira, H.; Carvalho, A.; Figueroa, J.A.L.; Zarmpas, P.; Cunha, C.; Akoumianaki, T.; Stylianou, K.; Deepe, G.S., Jr.; Samonis, G.; et al. Calcium sequestration by fungal melanin inhibits calcium–calmodulin signalling to prevent LC3-associated phagocytosis. Nat. Microbiol. 2018, 3, 791–803. [Google Scholar] [CrossRef]
- Nicola, A.M.; Albuquerque, P.; Martinez, L.R.; Dal-Rosso, R.A.; Saylor, C.; De Jesus, M.; Nosanchuk, J.D.; Casadevall, A. Macrophage Autophagy in Immunity to Cryptococcus neoformans and Candida albicans. Infect. Immun. 2012, 80, 3065–3076. [Google Scholar] [CrossRef] [Green Version]
- Panepinto, J.; Komperda, K.; Frases, S.; Park, Y.-D.; Djordjevic, J.T.; Casadevall, A.; Williamson, P.R. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 2009, 71, 1165–1176. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Godinho, R.M.C.; Zamith-Miranda, D.; Nimrichter, L. Traveling into Outer Space: Unanswered Questions about Fungal Extracellular Vesicles. PLoS Pathog. 2015, 11, e1005240. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Nakayasu, E.; Sobreira, T.J.P.; Choi, H.; Casadevall, A.; Nimrichter, L.; Nosanchuk, J.D. Antibody Binding Alters the Characteristics and Contents of Extracellular Vesicles Released by Histoplasma capsulatum. mSphere 2016, 1, e00085-15. [Google Scholar] [CrossRef] [Green Version]
- Casadevall, A.; Perfect, J.R. Cryptococcus Neoformans; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Pukkila-Worley, R.; Gerrald, Q.D.; Kraus, P.R.; Boily, M.-J.; Davis, M.J.; Giles, S.S.; Cox, G.M.; Heitman, J.; Alspaugh, J.A. Transcriptional Network of Multiple Capsule and Melanin Genes Governed by the Cryptococcus neoformans Cyclic AMP Cascade. Eukaryot. Cell 2005, 4, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Salas, S.D.; E Bennett, J.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front. Biosci. 1997, 2, e99–e107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tewari, R.P.; Williamson, P.R. Laccase Protects Cryptococcus neoformans from Antifungal Activity of Alveolar Macrophages. Infect. Immun. 1999, 67, 6034–6039. [Google Scholar] [CrossRef] [Green Version]
- Erb-Downward, J.R.; Noggle, R.M.; Williamson, P.R.; Huffnagle, G.B. The role of laccase in prostaglandin production by Cryptococcus neoformans. Mol. Microbiol. 2008, 68, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Nimrichter, L.; Casadevall, A.; Rodrigues, M.L. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun. Integr. Biol. 2008, 1, 37–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanio, A.; Desnos-Ollivier, M.; Dromer, F. Dynamics of Cryptococcus neoformans-Macrophage Interactions Reveal that Fungal Background Influences Outcome during Cryptococcal Meningoencephalitis in Humans. mBio 2011, 2, e00158-11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielska, E.; May, R.C. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016, 16, fov106. [Google Scholar] [CrossRef] [Green Version]
- Hansakon, A.; Ngamskulrungroj, P.; Angkasekwinai, P. Contribution of Laccase Expression to Immune Response against Cryptococcus gattii Infection. Infect. Immun. 2020, 88, e00712-19. [Google Scholar] [CrossRef]
- Mukaremera, L.; McDonald, T.R.; Nielsen, J.N.; Molenaar, C.J.; Akampurira, A.; Schutz, C.; Taseera, K.; Muzoora, C.; Meintjes, G.; Meya, D.B.; et al. The Mouse Inhalation Model of Cryptococcus neoformans Infection Recapitulates Strain Virulence in Humans and Shows that Closely Related Strains Can Possess Differential Virulence. Infect. Immun. 2019, 87, e00046-19. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Guo, Y.; Zhao, Y.; Xu, Y.; Peng, X.; Yang, Z.; Tao, R.; Huang, Y.; Xu, Y.; Chen, Y.; et al. Chemokine and Cytokine Cascade Caused by Skewing of the Th1-Th2 Balance Is Associated with High Intracranial Pressure in HIV-Associated Cryptococcal Meningitis. Mediat. Inflamm. 2019, 2019, 2053958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Jang, E.-H.; Lee, M.; Kim, S.-W.; Lee, Y.; Lee, K.-T.; Bahn, Y.-S. Unraveling Melanin Biosynthesis and Signaling Networks in Cryptococcus neoformans. mBio 2019, 10, e02267-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarasinghe, H.; Aceituno-Caicedo, D.; Cogliati, M.; Kwon-Chung, K.J.; Rickerts, V.; Velegraki, A.; Akcaglar, S.; Xu, J. Genetic Factors and Genotype-Environment Interactions Contribute to Variation in Melanin Production in the Fungal Pathogen Cryptococcus neoformans. Sci. Rep. 2018, 8, 9824. [Google Scholar] [CrossRef] [Green Version]
- Cordero, R.J.B.; Camacho, E.; Casadevall, A. Melanization in Cryptococcus neoformans Requires Complex Regulation. mBio 2020, 11, e03313-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.J.; Day, A.W. Fungal melanins: A review. Can. J. Microbiol. 1998, 44, 1115–1136. [Google Scholar] [CrossRef]
- Beardsley, J.; Sorrell, T.C.; Chen, S.C.-A. Central Nervous System Cryptococcal Infections in Non-HIV Infected Patients. J. Fungi 2019, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Ngamskulrungroj, P.; Himmelreich, U.; Breger, J.A.; Wilson, C.; Chayakulkeeree, M.; Krockenberger, M.B.; Malik, R.; Daniel, H.-M.; Toffaletti, D.; Djordjevic, J.T.; et al. The Trehalose Synthesis Pathway Is an Integral Part of the Virulence Composite for Cryptococcus gattii. Infect. Immun. 2009, 77, 4584–4596. [Google Scholar] [CrossRef] [Green Version]
- Petzold, E.W.; Himmelreich, U.; Mylonakis, E.; Rude, T.; Toffaletti, D.; Cox, G.M.; Miller, J.L.; Perfect, J.R. Characterization and Regulation of the Trehalose Synthesis Pathway and Its Importance in the Pathogenicity of Cryptococcus neoformans. Infect. Immun. 2006, 74, 5877–5887. [Google Scholar] [CrossRef] [Green Version]
- Levine, B. Eating oneself and uninvited guests: Autophagy-related pathways in cellular defense. Cell 2005, 120, 159–162. [Google Scholar]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.G.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Geng, J.; Klionsky, D.J. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. EMBO Rep. 2008, 9, 859–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firacative, C.; Duan, S.; Meyer, W. Galleria mellonella Model Identifies Highly Virulent Strains among All Major Molecular Types of Cryptococcus gattii. PLoS ONE 2014, 9, e105076. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Moreno, R.; El Khoury, J.B.; Idnurm, A.; Heitman, J.; Calderwood, S.B.; Ausubel, F.M.; Diener, A. Galleria mellonella as a Model System To Study Cryptococcus neoformans Pathogenesis. Infect. Immun. 2005, 73, 3842–3850. [Google Scholar] [CrossRef] [Green Version]
- Trevijano-Contador, N.; Zaragoza, O. Immune Response of Galleria mellonella against Human Fungal Pathogens. J. Fungi 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Bouklas, T.; Navarro, E.D.; Wang, X.; Fenster, M.; Fries, B.C. Characterization of the virulence of Cryptococcus neoformans strains in an insect model. Virulence 2015, 6, 809–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-L.; Lehman, V.N.; Lewit, Y.; Averette, A.F.; Heitman, J. Calcineurin Governs Thermotolerance and Virulence of Cryptococcus gattii. G3 Genes Genomes Genet. 2013, 3, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Solache, M.A.; Izquierdo-Garcia, D.; Smith, C.; Bergman, A.; Casadevall, A. Fungal Virulence in a Lepidopteran Model Is an Emergent Property with Deterministic Features. mBio 2013, 4, e00100-13. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, Y.; Xu, J. Phenotypic Plasticity in the Productions of Virulence Factors within and among Serotypes in the Cryptococcus neoformans Species Complex. Mycopathol. Et Mycol. Appl. 2021, 187, 65–83. [Google Scholar] [CrossRef]







| Age (years) | 42 ± 17.7 (mean ± std. dev.) |
| Gender | 68.3% male, 31.7% female |
| HIV infection status A | 68.3% positive, 29.3% negative, 2.4% unknown |
| CD4 count (cells per mm3) B | 71 ± 78.9 (median ± std. dev.) |
| Other risk factors C | 9.8% |
| Apparently immunocompetent D | 19.5% |
| Intracranial hypertension E | 48.6%—Yes; 10.8%—No; 40.6%—no information |
| Poor prognosis criteria F | 58.5% |
| Two-week mortality G | 30.8% |
| Ten-week mortality H | 41% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, H.R.; de Oliveira, G.P., Jr.; Frazão, S.d.O.; Gorgonha, K.C.d.M.; Rosa, C.P.; Garcez, E.M.; Lucas, J., Jr.; Correia, A.F.; de Freitas, W.F.; Borges, H.M.; et al. Faster Cryptococcus Melanization Increases Virulence in Experimental and Human Cryptococcosis. J. Fungi 2022, 8, 393. https://doi.org/10.3390/jof8040393
de Sousa HR, de Oliveira GP Jr., Frazão SdO, Gorgonha KCdM, Rosa CP, Garcez EM, Lucas J Jr., Correia AF, de Freitas WF, Borges HM, et al. Faster Cryptococcus Melanization Increases Virulence in Experimental and Human Cryptococcosis. Journal of Fungi. 2022; 8(4):393. https://doi.org/10.3390/jof8040393
Chicago/Turabian Stylede Sousa, Herdson Renney, Getúlio Pereira de Oliveira, Jr., Stefânia de Oliveira Frazão, Kaio César de Melo Gorgonha, Camila Pereira Rosa, Emãnuella Melgaço Garcez, Joaquim Lucas, Jr., Amabel Fernandes Correia, Waleriano Ferreira de Freitas, Higor Matos Borges, and et al. 2022. "Faster Cryptococcus Melanization Increases Virulence in Experimental and Human Cryptococcosis" Journal of Fungi 8, no. 4: 393. https://doi.org/10.3390/jof8040393
APA Stylede Sousa, H. R., de Oliveira, G. P., Jr., Frazão, S. d. O., Gorgonha, K. C. d. M., Rosa, C. P., Garcez, E. M., Lucas, J., Jr., Correia, A. F., de Freitas, W. F., Borges, H. M., Brito Alves, L. G. d., Paes, H. C., Trilles, L., Lazera, M. d. S., Teixeira, M. d. M., Pinto, V. L., Jr., Felipe, M. S. S., Casadevall, A., Silva-Pereira, I., ... Nicola, A. M. (2022). Faster Cryptococcus Melanization Increases Virulence in Experimental and Human Cryptococcosis. Journal of Fungi, 8(4), 393. https://doi.org/10.3390/jof8040393