Is Hyperdermium Congeneric with Ascopolyporus? Phylogenetic Relationships of Ascopolyporus spp. (Cordycipitaceae, Hypocreales) and a New Genus Neohyperdermium on Scale Insects in Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Isolation
2.2. Morphological Study
2.3. DNA Extraction, PCR and Sequencing
2.4. Sequence Alignment and Phylogenetic Analyses
3. Results
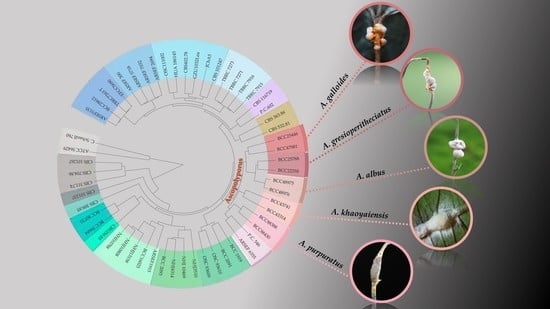
3.1. Molecular Phylogeny
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondo, T.; Gullan, P.J.; Williams, D.J. Coccidology. The study of scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Cienc. Tecnol. Agropecuaria 2008, 9, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Mansour, R.; Grissa-Lebdi, K.; Suma, P.; Mazzeo, G.; Russo, A. Key scale insects (Hemiptera: Coccoidea) of high economic importance in a Mediterranean area: Host plants, bio-ecological characteristics, natural enemies and pest management strategies—A review. Plant Prot. Sci. 2017, 53, 1–14. [Google Scholar]
- Dhami, M.K.; Weir, B.S.; Taylor, M.W.; Beggs, J.R. Diverse honeydew-consuming fungal communities associated with scale insects. PLoS ONE 2013, 8, e70316. [Google Scholar]
- Chomnunti, P.; Hongsanan, S.; Aguirre-Hudson, B.; Tian, Q.; Peršoh, D.; Dhami, M.K.; Alias, A.S.; Xu, J.; Liu, X.; Stadler, M.; et al. The sooty moulds. Fungal Divers. 2014, 66, 1–36. [Google Scholar] [CrossRef]
- Hongsanan, S.; Tian, Q.; Hyde, K.; Chomnunti, P. Two new species of sooty moulds, Capnodium coffeicola and Conidiocarpus plumeriae in Capnodiaceae. Mycosp 2015, 6, 814–824. [Google Scholar] [CrossRef]
- Henk, D.A.; Vilgalys, R. Molecular phylogeny suggests a single origin of insect symbiosis in the Pucciniomycetes with support for some relationships within the genus Septobasidium. Am. J. Bot. 2007, 94, 1515–1526. [Google Scholar] [CrossRef]
- Dao, H.T.; Beattie, G.A.C.; Rossman, A.Y.; Burgess, L.W.; Holford, P. Four putative entomopathogenic fungi of armoured scale insects on Citrus in Australia. Mycol. Prog. 2016, 15, 47. [Google Scholar] [CrossRef]
- Araújo, J.P.; Hughes, D.P. Diversity of entomopathogenic fungi: Which groups conquered the insect body? Adv. Genet. 2016, 94, 1–39. [Google Scholar]
- Xu, X.-L.; Zeng, Q.; Lv, Y.-C.; Jeewon, R.; Maharachchikumbura, S.S.; Wanasinghe, D.N.; Hyde, K.D.; Xiao, Q.-G.; Liu, Y.-G.; Yang, C.-L. Insight into the systematics of novel entomopathogenic fungi associated with armored scale insect, kuwanaspis howardi (Hemiptera: Diaspididae) in China. J. Fungi 2021, 7, 628. [Google Scholar] [CrossRef]
- Hywel-Jones, N.L.; Samuels, G.J. Three species of Hypocrella with large stromata pathogenic on scale insects. Mycologia 1998, 90, 36–46. [Google Scholar] [CrossRef]
- Sullivan, R.F.; Bills, G.F.; Hywel-Jones, N.L.; White, J.F. Hyperdermium: A new clavicipitalean genus for some tropical epibionts of dicotyledonous plants. Mycologia 2000, 92, 908–918. [Google Scholar] [CrossRef]
- Bischoff, J.F.; White, J.F. Torrubiella piperis sp. nov. (Clavicipitaceae, Hypocreales), a new teleomorph of the Lecanicillium complex. Stud. Mycol. 2004, 50, 89–94. [Google Scholar]
- Koroch, A.; Juliani, H.; Bischoff, J.; Lewis, E.; Bills, G.; Simon, J.; White, J.F. Examination of plant biotrophy in the scale insect parasitizing fungus Dussiella tuberiformis. Symbiosis 2004, 37, 267–280. [Google Scholar]
- Bischoff, J.F.; Chaverri, P.; White, J.F. Clarification of the host substrate of Ascopolyporus and description of Ascopolyporus philodendrus sp. nov. Mycologia 2005, 97, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Bischoff, J.F.; Evans, H.C.; Hodge, K.T. Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 2005, 97, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Liu, M.; Hodge, K. A monograph of the entomopathogenic genera Hypocrella, Moelleriella, and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae), and their aschersonia-like anamorphs in the Neotropics. Stud. Mycol. 2008, 60, 1–66. [Google Scholar] [CrossRef]
- Johnson, D.; Sung, G.H.; Hywel-Jones, N.L.; Luangsa-Ard, J.J.; Bischoff, J.F.; Kepler, R.M.; Spatafora, J.W. Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota). Mycol. Res. 2009, 113, 279–289. [Google Scholar] [CrossRef]
- Fan, J.-H.; Xie, Y.-P.; Xue, J.-L.; Xiong, Q.; Jiang, W.-J.; Zhang, Y.-J.; Ren, Z.-M. The strain HEB01 of Fusarium sp., a new pathogen that infects brown soft scale. Ann. Microbiol. 2014, 64, 333–341. [Google Scholar] [CrossRef]
- Luangsa-ard, J.J.; Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Khonsanit, A.; Wutikhun, T. Helicocollum, a new clavicipitalean genus pathogenic to scale insects (Hemiptera) in Thailand. Mycol. Prog. 2017, 16, 419–431. [Google Scholar] [CrossRef]
- Deng, J.; Yu, Y.; Wang, X.; Liu, Q.; Huang, X. The ubiquity and development-related abundance dynamics of Ophiocordyceps fungi in soft scale insects. Microorganisms 2021, 9, 404. [Google Scholar] [CrossRef]
- Szklarzewicz, T.; Michalik, K.; Grzywacz, B.; Kalandyk-Kołodziejczyk, M.; Michalik, A. Fungal Associates of Soft Scale Insects (Coccomorpha: Coccidae). Cells 2021, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Index Fungorum Database. Available online: http://www.indexfungorum.org/ (accessed on 5 December 2021).
- Wood, M. Webwatch: Observing Mushrooms. Fungi Magazine 1, 2. Available online: https://mushroomobserver.org/ (accessed on 27 December 2021).
- Möller, A. Phycomyceten und Ascomyceten. Untersuchungen aus Brasilien. Botanische Mittheilungen aus den Tropen 9; Fischer: Jena, Germany, 1901. [Google Scholar]
- Luangsa-ard, J.J.; Tasanathai, K.; Thanakitpipattana, D.; Khonsanit, A.; Stadler, M. Novel and interesting Ophiocordyceps spp. (Ophiocordycipitaceae, Hypocreales) with superficial perithecia from Thailand. Stud. Mycol. 2018, 89, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Khonsanit, A.; Lamlertthon, S.; Luangsa-ard, J.J. New species in Aciculosporium, Shimizuomyces and a new genus Morakotia associated with plants in Clavicipitaceae from Thailand. FUSE 2021, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Khonsanit, A.; Noisripoom, W.; Mongkolsamrit, S.; Phosrithong, N.; Luangsa-ard, J.J. Five new species of Moelleriella infecting scale insects (Coccidae) in Thailand. Mycol. Prog. 2021, 20, 847–867. [Google Scholar] [CrossRef]
- Heritage, J.; Evans, E.G.V.; Killington, R. Introductory Microbiology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- The Royal Horticultural Society. The Fifth Edition Published by The Royal Horticultural Society; Vincent Square: London, UK, 2007. [Google Scholar]
- Thanakitpipattana, D.; Tasanathai, K.; Mongkolsamrit, S.; Khonsanit, A.; Lamlertthon, S.; Luangsa-Ard, J.J. Fungal pathogens occurring on Orthopterida in Thailand. Persoonia 2020, 44, 140. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cyptococcus species. J. Bacteriol. Res. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.H.; Hyten, A.; Spatafora, J.W. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sarver, B.A.; Brandt, M.; Chang, D.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: A community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery; Association for Computing Machinery: New York, NY, USA, 2011; pp. 1–8. [Google Scholar]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J. MrModeltest v2. Program Distributed by the Author; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Wilgenbusch, J.C.; Swofford, D. Inferring evolutionary trees with PAUP. Curr. Protoc. Bioinform. 2003, 1, 6.4.1–6.4.28. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Han, Y.; Liang, Z.; Jin, D. Lecanicillium araneogenum sp. nov., a new araneogenous fungus. Phytotaxa 2017, 305, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Chiriví-Salomón, J.S.; Danies, G.; Restrepo, S.; Sanjuan, T. Lecanicillium sabanense sp. nov. (Cordycipitaceae) a new fungal entomopathogen of coccids. Phytotaxa 2015, 234, 63–74. [Google Scholar] [CrossRef]
- Rehner, S.A.; Minnis, A.M.; Sung, G.H.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; White, J.F. Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Mol. Ecol. 2007, 16, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Spatafora, J.W. Cordyceps cardinalis sp. nov., a new species of Cordyceps with an east Asian-eastern North American distribution. Mycologia 2004, 96, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Wutikhun, T.; Spatafora, J.W.; Luangsa-Ard, J.J. Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 2018, 110, 230–257. [Google Scholar] [CrossRef]
- Tasanathai, K.; Thanakitpipattana, D.; Noisripoom, W.; Khonsanit, A.; Kumsao, J.; Luangsa-ard, J.J. Two new Cordyceps species from a community forest in Thailand. Mycol. Prog. 2016, 15, 28. [Google Scholar] [CrossRef]
- Kepler, R.M.; Sung, G.H.; Ban, S.; Nakagiri, A.; Chen, M.J.; Huang, B.; Li, Z.; Spatafora, J.W. New teleomorph combinations in the entomopathogenic genus Metacordyceps. Mycologia 2012, 104, 182–197. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, Y.; Fan, Q.; Duan, D.E.; Zhang, G.D.; Dai, R.Q.; Dai, Y.D.; Zeng, W.B.; Chen, Z.H.; Li, D.D. Multigene phylogeny of the family Cordycipitaceae (Hypocreales): New taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers. 2020, 103, 1–46. [Google Scholar] [CrossRef]
- Humber, R.A. Evolution of entomopathogenicity in fungi. J. Invertebr. Pathol. 2008, 98, 262–266. [Google Scholar] [CrossRef]
- Torres, M.S.; Singh, A.P.; Vorsa, N.; White, J.F. An analysis of ergot alkaloids in the Clavicipitaceae (Hypocreales, Ascomycota) and ecological implication. Symbiosis 2008, 46, 11–19. [Google Scholar]
 represents species with pulvinate stromata while
represents species with pulvinate stromata while  represents species with flattened stromata.
represents species with flattened stromata.
 represents species with pulvinate stromata while
represents species with pulvinate stromata while  represents species with flattened stromata.
represents species with flattened stromata.






| Species | Strain | GenBank Accession No. | References | |||
|---|---|---|---|---|---|---|
| LSU | TEF1 | RPB1 | RPB2 | |||
| Akanthomyces aculeatus | HUA 186145 | MF416520 | MF416465 | – | – | [44] |
| Akanthomyces attenuatus | CBS 402.78 | AF339565 | EF468782 | EF468888 | EF468935 | [45] |
| Akanthomyces lecanii | CBS 101247 | AF339555 | DQ522359 | DQ522407 | DQ522466 | [45] |
| Akanthomyces neoaraneogenus | GZU1032Lea | KX845704 | KX845698 | KX845700 | KX845702 | [46] |
| Akanthomyces sabanense | ANDES-F 1024 | KC875225 | KC633266 | – | KC633249 | [47] |
| Akanthomyces tuberculatus | OSC 111002 | DQ518767 | DQ522338 | DQ522384 | DQ522435 | [45] |
| Ascopolyporus albus | BCC48975 | OL322048 | OL322035 | OL322056 | OL322065 | This study |
| Ascopolyporus albus | BCC48976 | OL322049 | OL322036 | OL322057 | OL322066 | This study |
| Ascopolyporus galloides | BCC25446 | OL322042 | OL322029 | OL322053 | OL322060 | This study |
| Ascopolyporus galloides | BCC47981 | OL322043 | OL322030 | OL322054 | OL322061 | This study |
| Ascopolyporus galloides | BCC48704 | OL322044 | OL322031 | OL322055 | OL322062 | This study |
| Ascopolyporus griseoperitheciatus | BCC22358 | OL322050 | OL322037 | – | OL322067 | This study |
| Ascopolyporus griseoperitheciatus | BCC25788 | OL322051 | OL322038 | OL322058 | OL322068 | This study |
| Ascopolyporus khaoyaiensis | BCC43314 | OL322052 | OL322039 | – | OL322069 | This study |
| Ascopolyporus khaoyaiensis | BCC43741 | OL322041 | OL322040 | – | OL322070 | This study |
| Ascopolyporus polychrous | P.C. 546 | DQ118737 | DQ118745 | DQ127236 | – | [15] |
| Ascopolyporus purpuratus | BCC88388 | OL322046 | OL322033 | – | OL322064 | This study |
| Ascopolyporus purpuratus | BCC88389 | OL322047 | OL322034 | – | – | This study |
| Ascopolyporus purpuratus | BCC88430 | OL322045 | OL322032 | OL322059 | OL322063 | This study |
| Ascopolyporus villosus | ARSEF 6355 | AY886544 | DQ118750 | DQ127241 | – | [14,15] |
| Beauveria bassiana | ARSEF 300 | – | AY531924 | HQ880831 | HQ880903 | [33,48] |
| Beauveria kipukae | ARSEF 7032 | – | HQ881005 | HQ880875 | HQ880947 | [48] |
| Beauveria staphylinidicola | ARSEF 5718 | EF468836 | EF468776 | EF468881 | – | [45] |
| Beauveria varroae | ARSEF 2694 | – | HQ881004 | HQ880874 | HQ880946 | [48] |
| Blackwellomyces cardinalis | OSC 93609 | AY184962 | DQ522325 | DQ522370 | DQ522422 | [49,50] |
| Blackwellomyces cardinalis | OSC 93610 | AY184963 | EF469059 | EF469088 | EF469106 | [45,50] |
| Blackwellomyces pseudomilitaris | BCC 1919 | MF416534 | MF416478 | – | MF416440 | [44] |
| Blackwellomyces pseudomilitaris | BCC 2091 | MF416535 | MF416479 | – | MF416441 | [44] |
| Cordyceps bifusispora | EFCC 5690 | EF468806 | EF468746 | EF468854 | EF468909 | [45] |
| Cordyceps lepidopterorum | TBRC 7263 | MF140699 | MF140819 | MF140768 | MF140792 | [51] |
| Cordyceps piperis | CBS 116719 | AY466442 | DQ118749 | DQ127240 | EU369083 | [15,17] |
| Cordyceps takaomontana | BCC28612 | FJ765252 | FJ765268 | – | – | [52] |
| Cordyceps tenuipes | ARSEF 5135 | JF415980 | JF416020 | JN049896 | JF416000 | [53] |
| Engyodontium aranearum | CBS 309.85 | AF339526 | DQ522341 | DQ522387 | DQ522439 | [49] |
| Gibellula leiopus | BCC 16025 | MF416548 | MF416492 | MF416649 | – | [44] |
| Gibellula pulchra | NHJ 10808 | EU369035 | EU369018 | EU369056 | EU369076 | [17] |
| Gibellula sp. | NHJ 10788 | EU369036 | EU369019 | EU369058 | EU369078 | [17] |
| Gibellula sp. | NHJ 13158 | EU369037 | EU369020 | EU369057 | EU369077 | [17] |
| Hevansia arachnophila | NHJ 10469 | EU369031 | EU369008 | EU369047 | – | [17] |
| Hevansia cinerea | NHJ 3510 | – | EU369009 | EU369048 | EU369070 | [17] |
| Hevansia nelumboides | BCC 2093 | MF416530 | MF416473 | – | MF416437 | [44] |
| Hevansia novoguineensis | NHJ 4314 | – | EU369012 | EU369051 | EU369071 | [17] |
| Hyperdermium caulium | AF242354 | AF242354 | – | – | – | [11] |
| Hyperdermium pulvinatum | P.C. 602 | DQ118738 | DQ118746 | DQ127237 | – | [15] |
| Lecanicillium antillanum | CBS 350.85 | AF339536 | DQ522350 | DQ522396 | DQ522450 | [45] |
| Lecanicillium psalliotae | CBS 363.86 | AF339559 | EF468784 | EF468890 | – | [45] |
| Lecanicillium psalliotae | CBS 532.81 | AF339560 | EF469067 | EF469096 | EF469112 | [45] |
| Neotorrubiella chinghridicola | BCC 39684 | MK632096 | MK632148 | MK632071 | MK632181 | [30] |
| Neotorrubiella chinghridicola | BCC 80733 | MK632097 | MK632149 | MK632072 | MK632176 | [30] |
| Samsoniella aurantia | TBRC 7271 | MF140728 | MF140846 | MF140791 | – | [51] |
| Samsoniella aurantia | TBRC 7273 | MF140726 | MF140844 | – | MF140816 | [51] |
| Samsoniella inthanonensis | TBRC 7915 | MF140725 | MF140849 | MF140790 | MF140815 | [51] |
| Samsoniella inthanonensis | TBRC 7916 | MF140724 | MF140848 | MF140789 | MF140814 | [51] |
| Outgroup | ||||||
| Flavocillium bifurcatum | YFCC 6101 | MN576781 | MN576951 | MN576841 | MN576897 | [54] |
| Lecanicillium sp. | CBS 639.85 | KM283801 | KM283824 | KM283843 | KM283865 | [54] |
| Name | Host | Stromata (mm) | Perithecia (µm) | Asci (µm) | Ascospores (µm) | Conidiogenous Cell (µm) | Conidia (µm) | References |
|---|---|---|---|---|---|---|---|---|
| Ascopolyporus albus | Scale insect, | pulvinate, | semi-immersed, | hyaline, cylindrical, | hyaline, filiform, | solitary, | enteroblastic, | This study |
| Epiphyte | subglobose to globose | obpyriform, | up to 250 × 3–4 | whole, multiseptate, | slightly curved, | fusiform to acerose, | ||
| white to pinkish white | 250–320 × 100–120 | 95–135 × 1 | cylindrical, | 1–4 septate, | ||||
| 3–6 | up to 120 × 1–2 | 8–30 × 2–3.5 | ||||||
| A. caulium | Scale insect, | crustose, subcircular | cylindrical, | cylindrical to | filiform, | phialidic, | enteroblastic, | Sullivan et al., 2000 |
| Epiphyte | yellow to orange, | 200–250 × 65–80 | slightly fusiform, | multiseptate, | sparse layer | cylindrical to fusiform, | ||
| 5–100 | 100–160 × 8–9 | extending | slightly truncate at end, | |||||
| to the length | aseptate: 5–7 × 1–1.5 | |||||||
| of ascus × 1 wide | 1–5 septate: 15–30 × 1.5–3 | |||||||
| A. galloides | Scale insect, | pulvinate, hemispherical, | semi-immersed, | hyaline, cylindrical, | hyaline, filiform, | solitary, | enteroblastic, | This study |
| Epiphyte | upper: white-yellowish white | obclavate, | 129–175 × 3–6 | whole, aseptate, | slightly curved, | fusiform to acerose, | ||
| lower: strong orange | 170–340 × 60–110 | 131–216 × 0.5 | cylindrical, | aseptate to 1–4 septate, | ||||
| 1–7 | 30–294 × 1–2 | 5–27 × 2–4 | ||||||
| A. griseoperitheciatus | Scale insect | pulvinate, irregular pulvinate | semi-immersed, | hyaline, cylindrical, | hyaline, filiform, | solitary, | enteroblastic, | This study |
| Epiphyte | to subglobose, 3–7 | obovoid, | 150–193 × 4–5 | whole, aseptate, | slightly curved, | fusiform to acerose, | ||
| upper: very pale violet to | 150–320 × 80–140 | extending to the | cylindrical, | aseptate to 1–2 septate, | ||||
| light purplish gray | length of ascus | acremonium-like, | 4–17 × 2–4 | |||||
| lower: vivid yellow to orange | 43–265 × 1–2 | |||||||
| A. khaoyaiensis | Scale insect, | flattened to convex, | semi-immersed, | hyaline, cylindrical, | hyaline, filiform, | solitary, | enteroblastic, | This study |
| Epiphyte | cylindrical to irregular shaped, | obclavate, | up to 215 × 3–4 | whole, aseptate, | slightly curved, | fusiform to acerose, | ||
| very pale violet to dark purple | 300–360 × 100–120 | 175–200 × 1 | cylindrical, | aseptate to 1–3 septate, | ||||
| 3–25 | acremonium-like, | 7–22 × 1.5–3 | ||||||
| up to 50 × 1–2 | ||||||||
| A. philodendrus | Scale insect, | subglobose, | immersed, | cylindrical, | filiform, | simple, | enteroblastic, | White et al., 2003 |
| Epiphyte | upper: sterile, red-purple | obclavate, | 90–140 × 3–5 | length of ascus | phialidic, | subcylindrical, | ||
| lower: fertile, white to tan | 200–300 × 40–80 | 30–60 × 1–3 | aseptate to 1–4 septate, | |||||
| 12–25 | 7–25 × 3–4 | |||||||
| A. polychrous | Scale insect, | polypore-like, | underside of stroma, | hyaline, | hyaline, filiform | NA | enteroblastic, oval, | Möller, 1901; |
| Epiphyte | bright-rusty red or | immersed, | cylindrical, | to spiroid, 300 × 1, | 1-multiseptate, | White et al., 2003 | ||
| white to yellow | narrow obclavate, | 500 × 4 | disarticulate into | 7–12 × 4–6 | ||||
| 40 | up to 750 | part-spores, 6 × 1 | ||||||
| A. purpuratus | Scale insect, | flattened to convex, | semi-immersed, ovoid, | hyaline, | hyaline, filiform, | solitary, | enteroblastic, | This study |
| Epiphyte | yellow to very pale, | 300–420 × 100–150 | cylindrical, | whole, aseptate, | slightly curved, | fusiform to acerose, | ||
| purple-violet, | up to 240 × | 100–220 × 1– | cylindrical, | aseptate, | ||||
| 5–12 × 3–8 | 2–4 | 1.5 | acremonium-like, | 5–18 × 1.5–3 | ||||
| up to 55 × 1–2 | ||||||||
| A. villosus | Scale insect, | white to pale yellow, | not produced | NA | NA | phialidic | enteroblastic, | White et al., 2003; Bischoff et al., 2005 |
| Epiphyte | 12–25 | subcylindrical, guttulate, | ||||||
| aseptate to 1–4 septate, | ||||||||
| 10–22 × 2–5 | ||||||||
| Neohyperdermium piperis | Scale insect, | pulvinate, subglobose | immersed, obpyriform | cylindrical, | filiform, | upright, | hyaline, subcylindrical, | Bischoff and White, 2004 |
| Epiphyte | to cylindrical, | to cymbiform, | 120–170 × 3–5 | disarticulating into | verticillate, | rarely subglobose, | ||
| white to yellow, 3–10 × 3–6 | 175–290 × 40–80 | part-spores, 4–9 × 1–2 | 150–400 | aseptate, 3–5 × 1–2 | ||||
| N. pulvinatum | Scale insect, | pulvinate, white to tan, | cone-shaped, | linear, | filiform, | phialidic, hyaline, | enteroblastic, subcylindrical, | Sullivan et al., 2000 |
| Epiphyte | 3–6 | 150–250 × 100–130 | 150–240 × 5–7 | multiseptate, | 1 to several septa, | slightly arcuate, | ||
| 130–225 × 1 | 40–100 × 1–3 | aseptate: 14–16 × 2.5–3 | ||||||
| 1–5 septate: 22–30 × 2.5–4 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanakitpipattana, D.; Mongkolsamrit, S.; Khonsanit, A.; Himaman, W.; Luangsa-ard, J.J.; Pornputtapong, N. Is Hyperdermium Congeneric with Ascopolyporus? Phylogenetic Relationships of Ascopolyporus spp. (Cordycipitaceae, Hypocreales) and a New Genus Neohyperdermium on Scale Insects in Thailand. J. Fungi 2022, 8, 516. https://doi.org/10.3390/jof8050516
Thanakitpipattana D, Mongkolsamrit S, Khonsanit A, Himaman W, Luangsa-ard JJ, Pornputtapong N. Is Hyperdermium Congeneric with Ascopolyporus? Phylogenetic Relationships of Ascopolyporus spp. (Cordycipitaceae, Hypocreales) and a New Genus Neohyperdermium on Scale Insects in Thailand. Journal of Fungi. 2022; 8(5):516. https://doi.org/10.3390/jof8050516
Chicago/Turabian StyleThanakitpipattana, Donnaya, Suchada Mongkolsamrit, Artit Khonsanit, Winanda Himaman, Janet Jennifer Luangsa-ard, and Natapol Pornputtapong. 2022. "Is Hyperdermium Congeneric with Ascopolyporus? Phylogenetic Relationships of Ascopolyporus spp. (Cordycipitaceae, Hypocreales) and a New Genus Neohyperdermium on Scale Insects in Thailand" Journal of Fungi 8, no. 5: 516. https://doi.org/10.3390/jof8050516
APA StyleThanakitpipattana, D., Mongkolsamrit, S., Khonsanit, A., Himaman, W., Luangsa-ard, J. J., & Pornputtapong, N. (2022). Is Hyperdermium Congeneric with Ascopolyporus? Phylogenetic Relationships of Ascopolyporus spp. (Cordycipitaceae, Hypocreales) and a New Genus Neohyperdermium on Scale Insects in Thailand. Journal of Fungi, 8(5), 516. https://doi.org/10.3390/jof8050516