Effect of Homogenized Callus Tissue on the Rheological and Mechanical Properties of 3D-Printed Food
Abstract
:1. Introduction
2. Results
2.1. Callus Characterization
2.2. Composition of CT
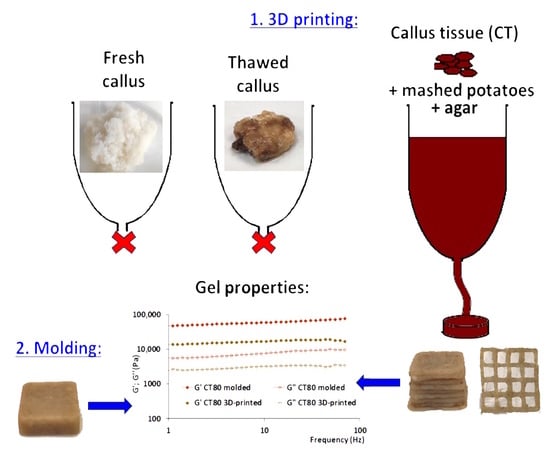
2.3. Effect of CT on the 3D Printability of Food Ink
2.4. Effect of CT on the Properties of the 3D-Printed Food Gels
2.5. Comparison of the Properties of the 3D-Printed and Molded Food Gels
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation and Characterizing CT
5.2. Rheological Characterization
5.3. The 3D-Printing Process
5.4. Measurement of Mechanical Properties
5.5. Scanning Electron Microscopy
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Key, T.; Papier, K.; Tong, T. Plant-based diets and long-term health: Findings from the EPIC-Oxford study. Proc. Nutr. Soc. 2022, 81, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, E.; Lille, M.; Silventoinen, P.; Nygren, H.; Seppänen-Laakso, T.; Mikkelson, A.; Aura, A.-M.; Heiniö, R.-L.; Nohynek, L.; Puupponen-Pimiä, R.; et al. Plant cells as food—A concept taking shape. Food Res. Int. 2018, 107, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, G.; Georgiev, V.; Pavlov, A. Recent applications of plant cell culture technology in cosmetics and foods. Eng. Life Sci. 2021, 21, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Gubser, G.; Vollenweider, S.; Eibl, D.; Eibl, R. Food ingredients and food made with plant cell and tissue cultures: State-of-the art and future trends. Eng. Life Sci. 2021, 21, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Aburto, A.; Trujillo-de Santiago, G.; Álvarez, M.M.; Chuck-Hernández, C. Advances and prospective applications of 3D food printing for health improvement and personalized nutrition. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5722–5741. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.; Paasi, J.; Pennanen, K.; Flores Ituarte, I.; Lille, M.; Partanen, J.; Sozer, N. Techno-Economic Prospects and Desirability of 3D Food Printing: Perspectives of Industrial Experts, Researchers and Consumers. Foods 2020, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Ortigoza, V.; Cuan-Urquizo, E. Towards the Development of 3D-Printed Food: A Rheological and Mechanical Approach. Foods 2022, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Ekonomou, S.I.; Hadnađev, M.; Gioxari, A.; Abosede, O.R.; Soe, S.; Stratakos, A.C. Advancing dysphagia-oriented multi-ingredient meal development: Optimising hydrocolloid incorporation in 3D printed nutritious meals. Food Hydrocoll. 2024, 147, 109300. [Google Scholar] [CrossRef]
- Outrequin, T.C.R.; Gamonpilas, C.; Siriwatwechakul, W.; Sreearunothai, P. Extrusion-based 3D printing of food biopolymers: A highlight on the important rheological parameters to reach printability. J. Food Eng. 2023, 342, 111371. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L. Formulated food inks for extrusion-based 3D printing of personalized foods: A mini review. Curr. Opin. Food Sci. 2022, 44, 100803. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Zang, Z.; Sun, X.; Tan, H.; Si, X.; Tian, J.; Teng, W.; Wang, J.; Liang, Q.; et al. 3D food printing: Applications of f-based materials in extrusion-based food printing. Crit. Rev. Food Sci. Nutr. 2021, 62, 7184–7198. [Google Scholar] [CrossRef] [PubMed]
- Belova, K.; Dushina, E.; Popov, S.; Zlobin, A.; Martinson, E.; Vityazev, F.; Litvinets, S. Enrichment of 3D-Printed k-Carrageenan Food Gel with Callus Tissue of Narrow-Leaved Lupin Lupinus angustifolius. Gels 2023, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Stanley, N.F. Agars. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.J., Williams, P.A., Eds.; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2006; pp. 217–238. [Google Scholar]
- Armisen, R.; Galatas, F. Agar. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2009; pp. 82–107. [Google Scholar]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Cueva, C.; Álvarez, M.D.; Herranz, B.; Bartolomé, B.; Moreno-Arribas, M.V.; Laguna, L. Influence of viscosity on the growth of human gut microbiota. Food Hydrocoll. 2018, 77, 163–167. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Álvarez, M.D.; Herranz, B.; Moreno-Arribas, M.V.; Laguna, L. Physical effects of dietary fibre on simulated luminal flow, studied by in vitro dynamic gastrointestinal digestion and fermentation. Food Funct. 2019, 10, 3452. [Google Scholar] [CrossRef] [PubMed]
- Dankar, I.; Haddarah, A.; Sepulcre, F.; Pujolà, M. Assessing Mechanical and Rheological Properties of Potato Puree: Effect of Different Ingredient Combinations and Cooking Methods on the Feasibility of 3D Printing. Foods 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.M.H.; Shiblee, N.I.; Ahmed, K.; Khosla, A.; Kawakami, M.; Furukawa, H. Rheological and mechanical properties of edible gel materials for 3D food printing technology. Heliyon 2020, 6, e05859. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Yang, C.-H. Dual extrusion 3D printing of mashed potatoes/strawberry juice gel. LWT Food Sci. Technol. 2018, 96, 589–596. [Google Scholar] [CrossRef]
- Mirazimi, F.; Saldo, J.; Sepulcre, F.; Gràcia, A.; Pujolà, M. Preparing a Personalized Meal by Using Soy, Cricket, and Egg Albumin Protein Based on 3D Printing. Foods 2022, 11, 2244. [Google Scholar] [CrossRef]
- Voon, S.L.; An, J.; Wong, G.; Zhang, Y.; Chua, C.K. 3D food printing: A categorised review of inks and their development. Virtual Phys. Prototyp. 2019, 14, 203–218. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [PubMed]
- Qiang, B.; Miao, J.; Phillips, N.; Wei, K.; Gao, Y. Recent Advances in the Tissue Culture of American Ginseng (Panax quinquefolius). Chem. Biodivers. 2020, 17, e2000366. [Google Scholar] [CrossRef] [PubMed]
- Pathi, K.M.; Sprink, T. From Petri Dish to Field: Plant Tissue Culture and Genetic Engineering of Oats for Improved Agricultural Outcomes. Plants 2023, 12, 3782. [Google Scholar] [CrossRef] [PubMed]
- An, Y.-J.; Guo, C.-F.; Zhang, M.; Zhong, Z.P. Investigation on characteristics of 3D printing using Nostoc sphaeroides biomass. Sci. Food Agric. 2019, 99, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Smirnov, V.; Paderin, N.; Khramova, D.; Chistiakova, E.; Vityazev, F.; Golovchenko, V. Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing. Gels 2022, 8, 708. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, M.; Bhandri, B. 3D printing of Cordyceps flower powder. J. Food Process. Eng. 2019, 42, e13179. [Google Scholar] [CrossRef]
- Keerthana, K.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Development of fiber-enriched 3D printed snacks from alternative foods: A study on button mushroom. J. Food Eng. 2020, 287, 110116. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, L.; Zou, Y.; Tong, Z.; Han, S.; Wang, S. 3D food printing: Main components selection by considering rheological properties. Crit. Rev. Food Sci. Nutr. 2018, 59, 2335–2347. [Google Scholar] [CrossRef]
- Liu, Y.; Yua, Y.; Liu, C.; Regenstein, J.M.; Liu, X.; Zhou, P. Rheological and mechanical behavior of milk protein composite gel for extrusion-based 3D food printing. LWT Food Sci. Technol. 2019, 102, 338–346. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Guo, C.; Zheng, W.; Cao, S.; Lu, H.; Mo, H.; Li, H. 3D Printing of Shiitake Mushroom Incorporated with Gums as Dysphagia Diet. Foods 2021, 10, 2189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Bhandari, B. Effect of gums on the rheological, microstructural and extrusion printing characteristics of mashed potatoes. Int. J. Biol. Macromol. 2018, 117, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.; Medina, J.; Molina, A.; Guti’errez, J.; Rodríguez, B.; Marín, R. Impact of viscoelastic and structural properties from starch-mango and starch-arabinoxylans hydrocolloids in 3D food printing. Addit. Manuf. 2021, 39, 101891. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Pandya, J.K.; McClements, D.J.; Lu, J.; Kinchla, A.J. Advancements in 3D food printing: A comprehensive overview of properties and opportunities. Crit. Rev. Food Sci. Nutr. 2022, 62, 4752–4768. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, J.H.; Park, S.M.; Lee, M.H.; Lee, I.W.; Doh, H.S.; Park, H.J. Effect of Hydrocolloids on Rheological Properties and Printability of Vegetable Inks for 3D Food Printing. J. Food Sci. 2018, 83, 2923. [Google Scholar] [CrossRef]
- Azam, R.S.M.; Zhang, M.; Bhandari, B.; Yang, C. Effect of different gums on features of 3D printed object Based on vitamin-D enriched orange concentrate. Food Biophys. 2018, 13, 250–262. [Google Scholar] [CrossRef]
- Gholamipour-Shirazi, A.; Norton, I.T.; Mills, T. Designing hydrocolloid based food-ink formulations for extrusion 3D printing. Food Hydrocoll. 2019, 95, 161–167. [Google Scholar] [CrossRef]
- Yang, F.; Cui, Y.; Guo, Y.; Yang, W.; Liu, X.; Liu, X. Internal structure and textural properties of a milk protein composite gel construct produced by three-dimensional printing. J. Food Sci. 2021, 86, 1917–1927. [Google Scholar] [CrossRef]
- Chena, J.; Mua, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubrug, E. Application of soy protein isolate and hydrocolloids based mixtures as promising food material in 3D food printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Kiumarsi, M.; Rafe, A.; Yeganehzad, S. Effect of Different Bulk Sweeteners on the Dynamic Oscillatory and Shear Rheology of Chocolate. Appl. Rheol. 2017, 27, 64123. [Google Scholar]
- Thakur, R.; Yadav, B.K.; Goyal, N. An Insight into Recent Advancement in Plant- and Algae-Based Functional Ingredients in 3D Food Printing Ink Formulations. Food Bioprocess Technol. 2023, 16, 1919–1942. [Google Scholar] [CrossRef]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsa-Kortelainen, E.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Johansson, M.; Nilsson, K.; Knab, F.; Langton, M. Faba Bean Fractions for 3D Printing of Protein-, Starch- and Fibre-Rich Foods. Processes 2022, 10, 466. [Google Scholar] [CrossRef]
- Derossi, A.; Husain, A.; Caporizzi, R.; Severini, C. Manufacturing personalized food for people uniqueness. An overview from traditional to emerging technologies. Crit. Rev. Food Sci. Nutr. 2019, 60, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Alghooneh, A.; Razavi, S.M.A.; Kasapis, S. Classification of hydrocolloids based on small amplitude oscillatory shear, large amplitude oscillatory shear, and textural properties. J. Texture Stud. 2019, 50, 520–538. [Google Scholar] [CrossRef] [PubMed]
- Strother, H.; Moss, R.; McSweeney, M.B. Comparison of 3D printed and molded carrots produced with gelatin, guar gum and xanthan gum. J. Texture Stud. 2020, 51, 852–860. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef]
- Holdsworth, S.D. Applicability of rheological models to the interpretation of flow and processing behavior of fluid food products. J. Texture Stud. 1971, 2, 393–418. [Google Scholar] [CrossRef]
- Ramkumar, D.H.S.; Bhattacharya, M. Relaxation behavior and the application of integral constitutive equations to wheat dough. J. Texture Stud. 1996, 27, 517–544. [Google Scholar] [CrossRef]
- Gabriele, D.G.; Migliori, M.; Sanzo, R.D.; Rossi, C.O.; Ruffolo, S.A.; Cindio, B. Characterization of dairy emulsions by NMR and rheological techniques. Food Hydrocoll. 2009, 23, 619–628. [Google Scholar] [CrossRef]
- Ge, S.; Liu, Q.; Li, M.; Liu, J.; Lu, H.; Li, F.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers. Food Hydrocoll. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Mojarrad, L.S.; Rafe, A.; Sadeghian, A.; Niazmand, R. Effects of high amylose corn starch and microbial transglutaminase on the textural and microstructural properties of wheat flour composite gels at high temperatures. J. Texture Stud. 2017, 48, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.W.Y.; Ikeda, S. The Young’s Modulus, Fracture Stress, and Fracture Strain of Gellan Hydrogels Filled with Whey Protein Microparticles. J. Food Sci. 2017, 82, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Paderin, N.; Chistiakova, E.; Ptashkin, D. Serosal Adhesion Ex Vivo of Hydrogels Prepared from Apple Pectin Cross-Linked with Fe3+ Ions. Int. J. Mol. Sci. 2023, 24, 1248. [Google Scholar] [CrossRef]










| Callus | Weight (g) | Volume (cm3) | Density (g/cm3) | MC (%) | pH |
|---|---|---|---|---|---|
| Fresh | 3.2 ± 0.6 | 3.6 ± 0.9 | 0.9 ± 0.1 | 97.1 ± 1.1 | 5.98 ± 0.00 |
| Frozen/Thawed | 2.0 ± 0.1 * | 2.8 ± 0.4 * | 0.7 ± 0.1 * | 95.2 ± 0.7 * | 4.66 ± 0.00 * |
| Parameters | Fresh | Frozen/Thawed | CT | ||
|---|---|---|---|---|---|
| k′ (Pa·s) | 22,464 ± 3032 a | 20,734 ± 13,082 a | 13,953 ± 1341 a | ||
| k″ (Pa·s) | 3026 ± 228 a | 2881 ± 1729 a | 1837 ± 170 a | ||
| A | 24,273 ± 2760 a | 21,058 ± 13,082 a | 13,937 ± 1395 a | ||
| k″/k′ | 7.45 ± 0.75 a | 7.11 ± 0.51 a | 7.59 ± 0.25 a | ||
| η*s | 3727 ± 871 a | 3265 ± 1895 a | 2187 ± 207 a | ||
| n’ | 0.07 ± 0.03 a | 0.11 ± 0.02 b | 0.11 ± 0.01 b | ||
| n″ | 0.06 ± 0.02 a | 0.17 ± 0.02 b | 0.18 ± 0.01 b | ||
| z | 21.21 ± 4.95 a | 9.31 ± 1.37 b | 8.71 ± 0.79 b | ||
| Frequency (Hz) | 0.54 | G′ (kPa) | 23,818 ± 3488 a | 19,378 ± 13,032 a,b | 12,083 ± 2108 b |
| G″ (kPa) | 7208 ± 2118 a | 3847 ± 2534 b | 1749 ± 285 b | ||
| 1.11 | G′ (kPa) | 22,872 ± 6283 a | 21,329 ± 13,210 a | 13,411 ± 2300 a | |
| G″ (kPa) | 3380 ± 620 a | 2917 ± 1799 a,b | 1753 ± 291 b | ||
| 10.50 | G′ (kPa) | 25,083 ± 2472 a | 26,616 ± 16,587 a | 16,886 ± 2948 a | |
| G″ (kPa) | 3328 ± 932 a | 4075 ± 2550 a | 2576 ± 453 a | ||
| 30.60 | G′ (kPa) | 27,458 ± 4922 a | 30,178 ± 18,911 a | 19,106 ± 3445 a | |
| G″ (kPa) | 3637 ± 1604 a | 5216 ± 3474 a | 3263 ± 647 a | ||
| 50.00 | G′ (kPa) | 28,846 ± 5543 a | 33,179 ± 21,298 a | 20,333 ± 3765 a | |
| G″ (kPa) | 3599 ± 872 a | 5359 ± 2775 a | 3548 ± 794 a | ||
| Polysaccharide | UA a | Gal a | Xyl a | Glc a | Rha a | Ara a | OMe b |
|---|---|---|---|---|---|---|---|
| LA-1 | 47.3 | 29.5 | 2.0 | 5.0 | 1.0 | 13.8 | 0.7 |
| LA-2 | 90.9 | 2.2 | 0.2 | 0.6 | 1.8 | 4.2 | 0.8 |
| Parameters | 3D-Printed Samples | Molded Samples | ||
|---|---|---|---|---|
| CT0 | CT80 | CT0 | CT80 | |
| Hardness (N) | 0.32 ± 0.03 | 0.21 ±0.03 a | 0.38 ±0.04 # | 0.21 ± 0.01 b |
| Young’s Modulus (kPa) | 728 ± 68 | 511 ± 89 a | 1612 ± 124 # | 922 ± 57 b |
| Elasticity (mm) | 2.4 ± 0.3 | 2.8 ±0.9 a | 0.85 ± 0.07 # | 0.76 ± 0.04 b,# |
| Parameters | 3D-Printed Sample | Molded Sample | ||
|---|---|---|---|---|
| CT0 | CT80 | CT0 | CT80 | |
| τ0 (Pa) | 168 ± 42 | 30 ± 9 a | 400 ± 57 # | 331 ± 40 # |
| K | 5116 ± 894 | 2484 ± 181 a | 7351 ± 681 # | 7204 ± 315 # |
| n | −0.955 | −0.925 | −0.913 | −0.879 |
| (A) Strength of linkage | ||||
| G′LVE (Pa) | 49,774 ± 3175 | 6303 ± 605 a | 42,518 ± 999 | 41,630 ± 2703 # |
| G*LVE | 50,353 ± 1966 | 6616 ± 362 a | 43,331 ± 926 # | 42,441 ± 2118 # |
| tan [δ]AF | 0.17 ± 0.01 | 0.05 ± 0.01 a | 0.08 ± 0.04 # | 0.05 ± 0.01 |
| (B) Number of linkages | ||||
| G*max/G*LVE | 1.06 ± 0.01 | 1.04 ± 0.02 | 1.02 ± 0.01 # | 1.05 ± 0.01 b |
| τFr (Pa) | 13,241 ± 2329 | 1621 ± 711 a | 7820 ± 1725 a,# | 12,969 ± 1510 b,# |
| (C) Timescale of junction zone | ||||
| tan [δ]LVE | 0.14 ± 0.02 | 0.19 ± 0.06 | 0.18 ± 0.04 | 0.15 ± 0.03 # |
| γL (%) | 0.16 ± 0.03 | 0.24 ± 0.04 | 0.20 ± 0.05 | 0.26 ± 0.03 |
| γFr (%) | 0.84 ± 0.09 | 1.34 ± 0.21 a | 3.10 ± 0.68 # | 1.85 ± 0.34 b,# |
| Ink Formulation | Agar, g | H2O, mL | MP, g | CT, g |
|---|---|---|---|---|
| CT0 | 3 | 100 | 20 | 0 |
| CT80 | 20 | 80 | ||
| CT85 | 15 | 85 | ||
| CT75 | 25 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dushina, E.; Popov, S.; Zlobin, A.; Martinson, E.; Paderin, N.; Vityazev, F.; Belova, K.; Litvinets, S. Effect of Homogenized Callus Tissue on the Rheological and Mechanical Properties of 3D-Printed Food. Gels 2024, 10, 42. https://doi.org/10.3390/gels10010042
Dushina E, Popov S, Zlobin A, Martinson E, Paderin N, Vityazev F, Belova K, Litvinets S. Effect of Homogenized Callus Tissue on the Rheological and Mechanical Properties of 3D-Printed Food. Gels. 2024; 10(1):42. https://doi.org/10.3390/gels10010042
Chicago/Turabian StyleDushina, Elena, Sergey Popov, Andrey Zlobin, Ekaterina Martinson, Nikita Paderin, Fedor Vityazev, Kseniya Belova, and Sergey Litvinets. 2024. "Effect of Homogenized Callus Tissue on the Rheological and Mechanical Properties of 3D-Printed Food" Gels 10, no. 1: 42. https://doi.org/10.3390/gels10010042
APA StyleDushina, E., Popov, S., Zlobin, A., Martinson, E., Paderin, N., Vityazev, F., Belova, K., & Litvinets, S. (2024). Effect of Homogenized Callus Tissue on the Rheological and Mechanical Properties of 3D-Printed Food. Gels, 10(1), 42. https://doi.org/10.3390/gels10010042