Fabrication of Sodium Trimetaphosphate-Based PEDOT:PSS Conductive Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
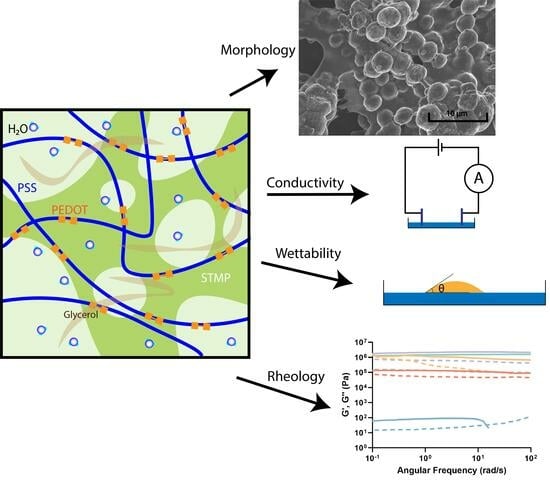
2.1. Hydrogel Morphology
2.2. Conductivity
2.3. Wettability Analysis
2.4. Rheology Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Conductive Hydrogel Formulations
4.3. Morphological Analysis
4.4. Conductivity Measurement
4.5. Hydrophilicity Analysis
4.6. Rheological Measurement
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Song, S.; McConnell, K.W.; Amores, D.; Levinson, A.; Vogel, H.; Quarta, M.; Rando, T.A.; George, P.M. Electrical stimulation of human neural stem cells via conductive polymer nerve guides enhances peripheral nerve recovery. Biomaterials 2021, 275, 120982. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.X.; Yan, J.; Quinn, D.; Dong, P.; Sawyer, S.W.; Soman, P. Fabrication of conductive gelatin methacrylate–polyaniline hydrogels. Acta Biomater. 2016, 33, 122–130. [Google Scholar] [CrossRef]
- George, P.M.; Song, S. Conductive polymer scaffolds to improve neural recovery. Neural Regen. Res. 2017, 12, 1976–1978. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Song, S.; Amores, D.; Chen, C.; McConnell, K.; Oh, B.; Poon, A.; George, P.M. Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds. Sci. Rep. 2019, 9, 19565. [Google Scholar] [CrossRef] [PubMed]
- Bates, N.M.; Puy, C.; Jurney, P.L.; McCarty, O.J.T.; Hinds, M.T. Evaluation of the Effect of Crosslinking Method of Poly(Vinyl Alcohol) Hydrogels on Thrombogenicity. Cardiovasc. Eng. Technol. 2020, 11, 448–455. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Lemos, P.V.F.; Opretzka, L.C.F.; Almeida, L.S.; Cardoso, L.G.; da Silva, J.B.A.; de Souza, C.O.; Villarreal, C.F.; Druzian, J.I. Preparation and characterization of C-phycocyanin coated with STMP/STPP cross-linked starches from different botanical sources. Int. J. Biol. Macromol. 2020, 159, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, P.; Liang, F.; Zhang, J.; Yuan, J.; Yin, M. A Nano-Silver Loaded PVA/Keratin Hydrogel With Strong Mechanical Properties Provides Excellent Antibacterial Effect for Delayed Sternal Closure. Front. Bioeng. Biotechnol. 2021, 9, 733980. [Google Scholar] [CrossRef]
- George, P.M.; Saigal, R.; Lawlor, M.W.; Moore, M.J.; LaVan, D.A.; Marini, R.P.; Selig, M.; Makhni, M.; Burdick, J.A.; Langer, R.; et al. Three-dimensional conductive constructs for nerve regeneration. J. Biomed. Mater. Res. A 2009, 91A, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wei, S.; Song, L.; Liu, H.; Wang, T. Conductive Hydrogels—A Novel Material: Recent Advances and Future Perspectives. J. Agric. Food Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Park, K.; Choi, H.; Son, D.; Shin, M. Self-Healing, Stretchable, Biocompatible, and Conductive Alginate Hydrogels through Dynamic Covalent Bonds for Implantable Electronics. Polymers 2021, 13, 1133. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, J.; Xu, B.; Liu, X.; Wang, H.; Wang, W.; Wang, Q.; Liu, W. A robust, highly stretchable supramolecular polymer conductive hydrogel with self-healability and thermo-processability. Sci. Rep. 2017, 7, 41566. [Google Scholar] [CrossRef]
- Khan, B.; Abdullah, S.; Khan, S. Current Progress in Conductive Hydrogels and Their Applications in Wearable Bioelectronics and Therapeutics. Micromachines 2023, 14, 1005. [Google Scholar] [CrossRef]
- Wu, C.; Liu, A.; Chen, S.; Zhang, X.; Chen, L.; Zhu, Y.; Xiao, Z.; Sun, J.; Luo, H.; Fan, H. Cell-Laden Electroconductive Hydrogel Simulating Nerve Matrix To Deliver Electrical Cues and Promote Neurogenesis. ACS Appl. Mater. Interfaces 2019, 11, 22152–22163. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Omer, S.A.; McKnight, K.H.; Young, L.I.; Song, S. Stimulation strategies for electrical and magnetic modulation of cells and tissues. Cell Regen. 2023, 12, 21. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Song, S.; Kang, J.; Tsao, Y.; Chen, S.; Mottini, V.; McConnell, K.; Xu, W.; Zheng, Y.-Q.; et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 2020, 38, 1031–1036. [Google Scholar] [CrossRef]
- Berggren, M.; Głowacki, E.D.; Simon, D.T.; Stavrinidou, E.; Tybrandt, K. In Vivo Organic Bioelectronics for Neuromodulation. Chem. Rev. 2022, 122, 4826–4846. [Google Scholar] [CrossRef]
- Li, C. Towards conductive hydrogels in e-skins: A review on rational design and recent developments. RSC Adv. 2021, 11, 33835–33848. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Rubio, M.C.; Carsi, M.; Ortiz-Serna, P.; Sanchis, M.J.; Garg, A.K.; Oliveira, J.M.; Koffler, J.; Collins, M.N. Electroconductive PEDOT nanoparticle integrated scaffolds for spinal cord tissue repair. Biomater. Res. 2022, 26, 63. [Google Scholar] [CrossRef] [PubMed]
- Testore, D.; Zoso, A.; Kortaberria, G.; Sangermano, M.; Chiono, V. Electroconductive Photo-Curable PEGDA-Gelatin/PEDOT:PSS Hydrogels for Prospective Cardiac Tissue Engineering Application. Front. Bioeng. Biotechnol. 2022, 10, 897575. [Google Scholar] [CrossRef]
- Antipova, C.G.; Parunova, Y.M.; Vishnevskaya, M.V.; Krasheninnikov, S.V.; Lukanina, K.I.; Grigoriev, T.E.; Chvalun, S.N.; Gotovtsev, P.M. Biomechanical behaviour of PEDOT:PSS-based hydrogels as an electrode for stent integrated enzyme biofuel cells. Heliyon 2022, 8, e09218. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.; Son, D.; Shin, M. Conductive and Adhesive Granular Alginate Hydrogels for On-Tissue Writable Bioelectronics. Gels 2023, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, e1806133. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J.M. In Vitro and In Vivo Evaluation of PEDOT Microelectrodes for Neural Stimulation and Recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Furlani, F.; Montanari, M.; Sangiorgi, N.; Saracino, E.; Campodoni, E.; Sanson, A.; Benfenati, V.; Tampieri, A.; Panseri, S.; Sandri, M. Electroconductive and injectable hydrogels based on gelatin and PEDOT:PSS for a minimally invasive approach in nervous tissue regeneration. Biomater. Sci. 2022, 10, 2040–2053. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Lee, S.-J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Roshanbinfar, K.; Vogt, L.; Greber, B.; Diecke, S.; Boccaccini, A.R.; Scheibel, T.; Engel, F.B. Electroconductive Biohybrid Hydrogel for Enhanced Maturation and Beating Properties of Engineered Cardiac Tissues. Adv. Funct. Mater. 2018, 28, 1803951. [Google Scholar] [CrossRef]
- Guan, S.; Wang, Y.; Xie, F.; Wang, S.; Xu, W.; Xu, J.; Sun, C. Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation. Molecules 2022, 27, 8326. [Google Scholar] [CrossRef]
- Mantione, D.; Del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef]
- Gotovtsev, P.M.; Badranova, G.U.; Zubavichus, Y.V.; Chumakov, N.K.; Antipova, C.G.; Kamyshinsky, R.A.; Presniakov, M.Y.; Tokaev, K.V.; Grigoriev, T.E. Electroconductive PEDOT:PSS-based hydrogel prepared by freezing-thawing method. Heliyon 2019, 5, e02498. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Kong, C.; Yang, C.; Yin, L.; Jeerapan, I.; Pu, F.; Zhang, X.; Yang, S.; Yang, Z. Wearable, stable, highly sensitive hydrogel–graphene strain sensors. Beilstein J. Nanotechnol. 2019, 10, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, C.; Yang, L.; Ao, X. Highly Stretchable, Self-Adhesive, Antidrying Ionic Conductive Organohydrogels for Strain Sensors. Molecules 2023, 28, 2817. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, Y.; Wang, L.; Ge, X.; Fu, M.; Wang, P.; Wang, Q. High-Strength Nanocomposite Hydrogels with Swelling-Resistant and Anti-Dehydration Properties. Polymers 2018, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Lack, S.; Dulong, V.; Picton, L.; Le Cerf, D.; Condamine, E. High-resolution nuclear magnetic resonance spectroscopy studies of polysaccharides crosslinked by sodium trimetaphosphate: A proposal for the reaction mechanism. Carbohydr. Res. 2007, 342, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-L.; Ansari, S.; Estevez, L.; Hayrapetyan, S.; Giannelis, E.P.; Lai, H.-M. Preparation and properties of biodegradable starch–clay nanocomposites. Carbohydr. Polym. 2010, 79, 391–396. [Google Scholar] [CrossRef]
- Noulis, K.; Frangopoulos, T.; Arampatzidou, A.; Tsekmes, L.; Marinopoulou, A.; Goulas, A.; Karageorgiou, V. Sodium Trimetaphosphate Crosslinked Starch Films Reinforced with Montmorillonite. Polymers 2023, 15, 3540. [Google Scholar] [CrossRef] [PubMed]
- Lack, S.; Dulong, V.; Le Cerf, D.; Picton, L.; Argillier, J.F.; Muller, G. Hydrogels Based on Pullulan Crosslinked with sodium trimetaphosphate (STMP): Rheological study. Polym. Bull. 2004, 52, 429–436. [Google Scholar] [CrossRef]
- Santamaría, E.; de Barros, L.A.; González, C.; Maestro, A. Rheological Study of the Formation of Pullulan Hydrogels and Their Use as Carvacrol-Loaded Nanoemulsion Delivery Systems. Gels 2023, 9, 644. [Google Scholar] [CrossRef]
- Samoila, I.; Dinescu, S.; Pircalabioru, G.G.; Marutescu, L.; Fundueanu, G.; Aflori, M.; Constantin, M. Pullulan/Poly(Vinyl Alcohol) Composite Hydrogels for Adipose Tissue Engineering. Materials 2019, 12, 3220. [Google Scholar] [CrossRef]
- Rafe, A.; Razavi, S.M.A. Scaling law, fractal analysis and rheological characteristics of physical gels cross-linked with sodium trimetaphosphate. Food Hydrocoll. 2017, 62, 58–65. [Google Scholar] [CrossRef]
- Trivedi, S.; Pamidi, V.; Fichtner, M.; Reddy, M.A. Ionically conducting inorganic binders: A paradigm shift in electrochemical energy storage. Green Chem. 2022, 24, 5620–5631. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, Y.; Xu, Q.; Liu, N.; Chen, P.; Zhou, Y.; Bai, Z. Rheological and ion-conductive properties of injectable and self-healing hydrogels based on xanthan gum and silk fibroin. Int. J. Biol. Macromol. 2020, 144, 473–482. [Google Scholar] [CrossRef]
- Xu, X.; He, C.; Luo, F.; Wang, H.; Peng, Z. Transparent, Conductive Hydrogels with High Mechanical Strength and Toughness. Polymers 2021, 13, 2004. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Bioinspired double network hydrogels: From covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater. Horiz. 2021, 8, 1173–1188. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Chen, Y.; Liu, Y.; Chen, G.; Guo, W.; Kang, X.; Pu, X.; Hu, W.; Wang, Z.L. A flexible triboelectric nanogenerator based on a super-stretchable and self-healable hydrogel as the electrode. Nanoscale 2020, 12, 12753–12759. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Z.; Chen, C.; Shi, K.; Zhang, L.; Ju, X.-J.; Wang, W.; Xie, R.; Chu, L.-Y. Reduced Graphene Oxide-Containing Smart Hydrogels with Excellent Electro-Response and Mechanical Properties for Soft Actuators. ACS Appl. Mater. Interfaces 2017, 9, 15758–15767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, L.; Zhang, H.; Li, Q.; Ma, N.; Zhang, X.; Ma, L. High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent. Molecules 2023, 28, 4690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Li, B.; Zhang, P.; Kan, L.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. One-Step Preparation of a Highly Stretchable, Conductive, and Transparent Poly(vinyl alcohol)–Phytic Acid Hydrogel for Casual Writing Circuits. ACS Appl. Mater. Interfaces 2019, 11, 32441–32448. [Google Scholar] [CrossRef]
- Ghosh, S.; Inganäs, O. Self-assembly of a conducting polymer nanostructure by physical crosslinking: Applications to conducting blends and modified electrodes. Synth. Met. 1999, 101, 413–416. [Google Scholar] [CrossRef]
- He, H.; Zhang, L.; Yue, S.; Yu, S.; Wei, J.; Ouyang, J. Enhancement in the Mechanical Stretchability of PEDOT:PSS Films by Compounds of Multiple Hydroxyl Groups for Their Application as Transparent Stretchable Conductors. Macromolecules 2021, 54, 1234–1242. [Google Scholar] [CrossRef]
- Zabihi, F.; Ahmadian-Yazdi, M.-R.; Eslamian, M. Fundamental Study on the Fabrication of Inverted Planar Perovskite Solar Cells Using Two-Step Sequential Substrate Vibration-Assisted Spray Coating (2S-SVASC). Nanoscale Res. Lett. 2016, 11, 71. [Google Scholar] [CrossRef]
- Joglekar, M.M.; Ghosh, D.; Anandan, D.; Yatham, P.; Jayant, R.D.; Nambiraj, N.A.; Jaiswal, A.K. Crosslinking of gum-based composite scaffolds for enhanced strength and stability: A comparative study between sodium trimetaphosphate and glutaraldehyde. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3147–3154. [Google Scholar] [CrossRef]
- Dulong, V.; Lack, S.; Le Cerf, D.; Picton, L.; Vannier, J.; Muller, G. Hyaluronan-based hydrogels particles prepared by crosslinking with trisodium trimetaphosphate. Synthesis and characterization. Carbohydr. Polym. 2004, 57, 1–6. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta BBA Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Özeren, H.D.; Wei, X.-F.; Nilsson, F.; Olsson, R.T.; Hedenqvist, M.S. Role of hydrogen bonding in wheat gluten protein systems plasticized with glycerol and water. Polymer 2021, 232, 124149. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, X.; Gan, J.; Geng, D.; Bian, X.; Cheng, Y.; Tang, N. A pH-sensitive hydrogel based on carboxymethylated konjac glucomannan crosslinked by sodium trimetaphosphate: Synthesis, characterization, swelling behavior and controlled drug release. Int. J. Biol. Macromol. 2023, 232, 123392. [Google Scholar] [CrossRef] [PubMed]
- Baltz, J. Media composition: Salts and osmolality. Methods Mol. Biol. Clifton NJ 2012, 912, 61–80. [Google Scholar]
- Kubota, N.; Fujii, S.; Tatsumoto, N.; Sano, T. Ionically conductive polymer gel electrolytes consisting of crosslinked methacrylonitrile and organic electrolyte. J. Appl. Polym. Sci. 2002, 83, 2655–2659. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Kalogeropoulos, T.; Thunberg, J.; Johannesson, S.; Hägg, D.; Enoksson, P.; Gatenholm, P. Enhanced growth of neural networks on conductive cellulose-derived nanofibrous scaffolds. Mater. Sci. Eng. C 2016, 58, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.Y.; Chang, N.Y.; Li, C.; Chan, V.; Hsieh, J.H.; Tsai, Y.-H.; Lin, T. Fabrication of Gelatin Nanofibers by Electrospinning—Mixture of Gelatin and Polyvinyl Alcohol. Polymers 2022, 14, 2610. [Google Scholar] [CrossRef]
- Cutiongco, M.F.; Goh, S.H.; Aid-Launais, R.; Le Visage, C.; Low, H.Y.; Yim, E.K. Planar and tubular patterning of micro and nano-topographies on poly(vinyl alcohol) hydrogel for improved endothelial cell responses. Biomaterials 2016, 84, 184–195. [Google Scholar] [CrossRef]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 66–75. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef]
- Filová, E.; Bullett, N.; Bačáková, L.; Grausová, L.; Haycock, J.; Hlučilová, J.; Klíma, J.; Shard, A. Regionally-selective cell colonization of micropatterned surfaces prepared by plasma polymerization of acrylic acid and 1,7-octadiene. Physiol. Res. 2009, 58, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and Characterization of Ulvan Polysaccharides-Based Hydrogel Films for Potential Wound Dressing Applications. Drug Des. Devel. Ther. 2021, 15, 4213–4226. [Google Scholar] [CrossRef]
- Dulong, V.; Forbice, R.; Condamine, E.; Le Cerf, D.; Picton, L. Pullulan–STMP hydrogels: A way to correlate crosslinking mechanism, structure and physicochemical properties. Polym. Bull. 2011, 67, 455–466. [Google Scholar] [CrossRef]
- Bagher, Z.; Atoufi, Z.; Alizadeh, R.; Farhadi, M.; Zarrintaj, P.; Moroni, L.; Setayeshmehr, M.; Komeili, A.; Kamrava, S.K. Conductive hydrogel based on chitosan-aniline pentamer/gelatin/agarose significantly promoted motor neuron-like cells differentiation of human olfactory ecto-mesenchymal stem cells. Mater. Sci. Eng. C 2019, 101, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Alvarez-Perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA Macroporous Hydrogels For Nerve Regeneration. Adv. Healthc. Mater. 2013, 2, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gaillez, M.P.; Rothe, R.; Hauser, S.; Voigt, D.; Pietzsch, J.; Zhang, Y. Conductive Hydrogels with Dynamic Reversible Networks for Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2100012. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Oh, T.H.; Kim, S.H.; Han, S.S.; Lee, S.J.; Lee, S.G.; Lee, Y.J.; Jang, S.S. Effect of solvent on electrical conductivity and gas sensitivity of PEDOT: PSS polymer composite films. J. Appl. Polym. Sci. 2015, 132, 42628. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef]
- Chen, Y.; Song, J.; Wang, S.; Liu, W. PVA-Based Hydrogels: Promising Candidates for Articular Cartilage Repair. Macromol. Biosci. 2021, 21, 2100147. [Google Scholar] [CrossRef]
- Oʼbrien, J.P.; Mackinnon, S.E.; MacLean, A.R.; Hudson, A.R.; Dellon, A.L.; Hunter, D.A. A model of chronic nerve compression in the rat. Ann. Plast. Surg. 1987, 19, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.M.; Abliz, E.; Wachrathit, K.; Krauthamer, V.; Shah, S.B. Biomechanical and functional variation in rat sciatic nerve following cuff electrode implantation. J. Neuroeng. Rehabil. 2014, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, X.; Liu, X.; Cui, R.; Wang, C.; Zhao, G.; Zhi, W.; Lu, M.; Duan, K.; Weng, J.; et al. 3D Bioprinting of shear-thinning hybrid bioinks with excellent bioactivity derived from gellan/alginate and thixotropic magnesium phosphate-based gels. J. Mater. Chem. B 2020, 8, 5500–5514. [Google Scholar] [CrossRef]
- Tuladhar, S.; Clark, S.; Habib, A. Tuning Shear Thinning Factors of 3D Bio-Printable Hydrogels Using Short Fiber. Materials 2023, 16, 572. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]





| Glycerol (vol. Ratio) | PEDOT:PSS (vol. Ratio) | STMP (vol. Ratio) | Conductivity (S/m) | θ (°) | Stiffness (Pa) | |
|---|---|---|---|---|---|---|
| NPN (Control) | 0 | 8 | 0 | (2.13 ± 0.58) × 105 | 47.16 ± 0.80 | (1.03 ± 0.07) × 106 |
| LPN | 1 | 8 | 0 | (1.98 ± 0.40) × 105 | 37.23 ± 0.51 | (7.33 ± 0.50) × 101 |
| NPL | 0 | 8 | 1 | (1.66 ± 0.37) × 105 | 23.96 ± 0.64 | (3.54 ± 0.28) × 106 |
| NPH | 0 | 8 | 15 | (1.78 ± 0.63) × 107 | 0.84 ± 0.16 | (4.09 ± 0.75) × 106 |
| LPH | 1 | 8 | 15 | (9.41 ± 2.83) × 104 | 0.11 ± 0.07 | (2.75 ± 0.31) × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, M.; Stoy, L.M.; Sun, J.; Opoku Amponsah, P.E.; Li, L.; Soto, M.; Song, S. Fabrication of Sodium Trimetaphosphate-Based PEDOT:PSS Conductive Hydrogels. Gels 2024, 10, 115. https://doi.org/10.3390/gels10020115
Reynolds M, Stoy LM, Sun J, Opoku Amponsah PE, Li L, Soto M, Song S. Fabrication of Sodium Trimetaphosphate-Based PEDOT:PSS Conductive Hydrogels. Gels. 2024; 10(2):115. https://doi.org/10.3390/gels10020115
Chicago/Turabian StyleReynolds, Madelyn, Lindsay M. Stoy, Jindi Sun, Prince Emmanuel Opoku Amponsah, Lin Li, Misael Soto, and Shang Song. 2024. "Fabrication of Sodium Trimetaphosphate-Based PEDOT:PSS Conductive Hydrogels" Gels 10, no. 2: 115. https://doi.org/10.3390/gels10020115
APA StyleReynolds, M., Stoy, L. M., Sun, J., Opoku Amponsah, P. E., Li, L., Soto, M., & Song, S. (2024). Fabrication of Sodium Trimetaphosphate-Based PEDOT:PSS Conductive Hydrogels. Gels, 10(2), 115. https://doi.org/10.3390/gels10020115