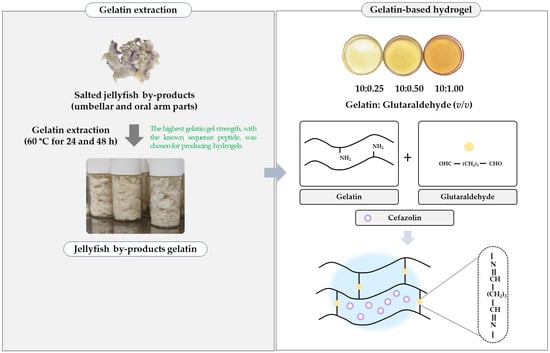
Exploring the Model of Cefazolin Released from Jellyfish Gelatin-Based Hydrogels as Affected by Glutaraldehyde
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quality of Jellyfish By-Product Gelatin Extracted from Different Parts of Jellyfish and Extraction Times
2.1.1. Gelatin Yield
2.1.2. Gel Strength
2.1.3. Viscosity
2.1.4. Gelling Properties
2.1.5. Fourier Transform Infrared (FTIR) Spectra
2.1.6. Protein Pattern
2.1.7. Identification of Proteins in Gelatin
2.2. Quality of Hydrogels
2.2.1. Gel Fraction and Porosity
2.2.2. Microstructure
2.2.3. Gel Swelling
2.2.4. In Vitro Drug Release
3. Conclusions
4. Materials and Methods
4.1. Preparation of Jellyfish By-Product Gelatin
4.2. Preparation of Gelatin-Based Hydrogels
4.3. Analysis of Gelatin Properties
4.3.1. Gelatin Yield (%)
4.3.2. Gel Strength
4.3.3. Viscosity
4.3.4. Thermal Stability
4.3.5. FTIR Spectroscopic Analysis
4.3.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.3.7. Protein Identification of Gelatin Using LC-MS/MS
4.4. Analysis of Gelatin-Based Hydrogel Properties
4.4.1. Gel Fraction
4.4.2. Gel Porosity
4.4.3. Microstructure
4.4.4. Gel swelling
4.4.5. In Vitro Drug Release
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BG | Commercial bovine gelatin |
| BGel | Bovine hydrogels |
| BGel0.25 | Bovine hydrogels with a ratio of commercial bovine gelatin: glutaraldehyde at 10:0.25 (v/v) |
| BGel0.50 | Bovine hydrogels with a ratio of commercial bovine gelatin: glutaraldehyde at 10:0.50 (v/v) |
| BGel1.00 | Bovine hydrogels with a ratio of commercial bovine gelatin: glutaraldehyde at 10:1.00 (v/v) |
| FG | Commercial fish gelatin |
| FGel | Fish hydrogels |
| FGel0.25 | Bovine hydrogels with a ratio of commercial fish gelatin: glutaraldehyde at 10:0.25 (v/v) |
| FGel0.50 | Bovine hydrogels with a ratio of commercial fish gelatin: glutaraldehyde at 10:0.50 (v/v) |
| FGel1.00 | Bovine hydrogels with a ratio of commercial fish gelatin: glutaraldehyde at 10:1.00 (v/v) |
| JGel | Jellyfish hydrogels |
| JGel0.25 | Jellyfish hydrogels with a ratio of gelatin extracted from the umbrella part of desalted jellyfish by-product for 24 h: glutaraldehyde at 10:0.25 (v/v) |
| JGel0.50 | Jellyfish hydrogels with a ratio of gelatin extracted from the umbrella part of desalted jellyfish by-product for 24 h: glutaraldehyde at 10:0.50 (v/v) |
| JGel1.00 | Jellyfish hydrogels with a ratio of gelatin extracted from the umbrella part of desalted jellyfish by-product for 24 h: glutaraldehyde at 10:1.00 (v/v) |
| WO24 | Gelatin extracted from the oral arm part of desalted jellyfish by-product for 24 h |
| WO48 | Gelatin extracted from the oral arm part of desalted jellyfish by-product for 48 h |
| WU24 | Gelatin extracted from the umbrella part of desalted jellyfish by-product for 24 h |
| WU48 | Gelatin extracted from the umbrella part of desalted jellyfish by-product for 48 h |
References
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart hydrogel-based DVDMS/bFGF nanohybrids for antibacterial phototherapy with multiple damaging sites and accelerated wound healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef]
- Grolman, J.M.; Singh, M.; Mooney, D.J.; Eriksson, E.; Nuutila, K. Antibiotic-containing agarose hydrogel for wound and burn care. J. Burn Care Res. 2019, 40, 900–906. [Google Scholar] [CrossRef]
- Kusaba, T. Safety and efficacy of cefazolin sodium in the management of bacterial infection and in surgical prophylaxis. Clin. Med. Ther. 2009, 1, 1607–1615. [Google Scholar] [CrossRef]
- Li, Z.; You, S.; Mao, R.; Xiang, Y.; Cai, E.; Deng, H.; Shen, J.; Qi, X. Architecting polyelectrolyte hydrogels with Cu-assisted polydopamine nanoparticles for photothermal antibacterial therapy. Mater. Today Bio 2022, 15, 100264. [Google Scholar] [CrossRef]
- Pan, F.; Giovannini, G.; Zhang, S.; Altenried, S.; Zuber, F.; Chen, Q.; Boesel, L.F.; Ren, Q. pH-responsive silica nanoparticles for the treatment of skin wound infections. Acta Biomater. 2022, 145, 172–184. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.J.; Liu, X.; Liu, Y.; Zhu, X.; Liu, X.; You, X. A Physically cross-linked sodium alginate–gelatin hydrogel with high mechanical strength. ACS Appl. Polym. Mater. 2021, 3, 3197–3205. [Google Scholar] [CrossRef]
- Tanaka, Y.; Gong, J.P.; Osada, Y. Novel hydrogels with excellent mechanical performance. Prog. Polym. Sci. 2005, 30, 1–9. [Google Scholar] [CrossRef]
- Distler, T.; McDonald, K.; Heid, S.; Karakaya, E.; Detsch, R.; Boccaccini, A.R. Ionically and enzymatically dual cross-linked oxidized alginate gelatin hydrogels with tunable stiffness and degradation behavior for tissue engineering. ACS Biomater. Sci. Eng. 2020, 6, 3899–3914. [Google Scholar] [CrossRef]
- Le, A.N.M.; Nguyen, T.T.; Ly, K.L.; Luong, T.D.; Ho, M.H.; Tran, N.M.P.; Dang, N.N.T.; Vo, T.V.; Tran, Q.N.; Nguyen, T.H. Modulating biodegradation and biocompatibility of in situ crosslinked hydrogel by the integration of alginate into N,O-carboxylmethyl chitosan—Aldehyde hyaluronic acid network. Polym. Degrad. Stabil. 2020, 180, 109270. [Google Scholar]
- Zhou, H.; Zhu, H.; Yang, X.; Zhang, Y.; Zhang, X.; Cui, K.; Shao, L.; Yao, J. Temperature/pH sensitive cellulose-based hydrogel: Synthesis, characterization, loading, and re-lease of model drugs for potential oral drug delivery. BioResources 2015, 10, 760–771. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-strength and high-toughness double-cross-linked cellulose hydrogels: A new strategy using sequential chemical and physical cross-linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Senapati, S.; Paliwal, P.; Krishnamurthy, S.; Hemalatha, S.; Maiti, P. Nano-particle-induced controlled drug delivery using chitosan-based hydrogel and scaffold: Application to bone regeneration. Mol. Pharm. 2018, 16, 327–338. [Google Scholar] [CrossRef]
- Zhu, Z.; Ling, S.; Yeo, J.; Zhao, S.; Tozzi, L.; Buehler, M.J.; Omenetto, F.; Li, C.; Kaplan, D.L. High-strength, durable all-silk fibroin hydrogels with versatile processability toward multifunctional applications. Adv. Funct. Mater. 2018, 28, 1704757. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Hamzeh, A.; Benjakul, S.; Sae-Leaw, T.; Sinthusamran, S. Effect of drying methods on gelatin from splendid squid (Loligo formosana) skins. Food Biosci. 2018, 26, 96–103. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in South East Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Muangrod, P.; Rungsardthong, V.; Vatanyoopaisarn, S.; Tamaki, Y.; Kuraya, E.; Thumthanaruk, B. Effect of wash cycle on physical and chemical properties of rehydrated jellyfish by-products and jellyfish protein powder. Sci. Eng. Health Stud. 2021, 15, 21030004. [Google Scholar]
- Ding, J.F.; Li, Y.Y.; Xu, J.J.; Su, X.R.; Gao, X.; Yue, F.P. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocoll. 2011, 25, 1350–1353. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Jia, A.; Zhang, Y.; Zhu, H.; Zhang, C.; Sun, Z.; Liu, C. Purification and characterization of angiotensin I converting enzyme inhibitory peptides from jellyfish Rhopilema esculentum. Food Res. Int. 2013, 50, 339–343. [Google Scholar] [CrossRef]
- Muangrod, P.; Charoenchokpanich, W.; Rungsardthong, V.; Vatanyoopaisarn, S.; Wonganu, B.; Roytrakul, S.; Thumthanaruk, B. Effect of pepsin hydrolysis on antioxidant activity of jellyfish protein hydrolysate. E3S Web Conf. 2021, 302, 02010. [Google Scholar] [CrossRef]
- Muangrod, P.; Charoenchokpanich, W.; Roytrakul, S.; Rungsardthong, V.; Vatanyoopaisarn, S.; Charoenlappanit, S.; Wonganu, B.; Thumthanaruk, B. Effect of pepsin on antioxidant and antibacterial activity of protein hydrolysate from salted jellyfish (Lobonema smithii and Rhopilema hispidum) by-products. E3S Web Conf. 2022, 355, 02013. [Google Scholar] [CrossRef]
- Rodsuwan, U.; Thumthanaruk, B.; Kerdchoechuen, O.; Laohakunjit, N. Functional properties of type A gelatin from jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 507–514. [Google Scholar]
- Cho, S.; Ahn, J.R.; Koo, J.S.; Kim, S.B. Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fish. Aquat. Sci. 2014, 17, 299–304. [Google Scholar] [CrossRef]
- Lueyot, A.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wonganu, B.; Wongsa-Ngasri, P.; Charoenlappanit, S.; Roytrakul, S.; Thumthanaruk, B. Influence of collagen and some proteins on gel properties of jellyfish gelatin. PLoS ONE 2021, 16, e0253254. [Google Scholar] [CrossRef] [PubMed]
- Lueyot, A.; Wonganu, B.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wongsa-Ngasri, P.; Roytrakul, S.; Charoenlappanit, S.; Wu, T.; Thumthanaruk, B. Improved jellyfish gelatin quality through ultrasound-assisted salt removal and an extraction process. PLoS ONE 2022, 17, e0276080. [Google Scholar] [CrossRef] [PubMed]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of extraction variables on the structure and physical properties of salmon gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Chiarelli, P.G.; Pegg, R.B.; Kumar, G.D.; Solval, K.M. Exploring the feasibility of developing novel gelatin powders from salted, dried cannonball jellyfish (Stomolophus meleagris). Food Biosci. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, R.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Effect of extraction methods on the structural characteristics, functional properties, and emulsion stabilization ability of Tilapia skin gelatins. Food Chem. 2020, 328, 127114. [Google Scholar] [CrossRef] [PubMed]
- Tümerkan, E.T.A.; Cansu, Ü.; Boran, G.; Regenstein, J.M.; Özoğul, F. Physiochemical and functional properties of gelatin obtained from tuna, frog and chicken skins. Food Chem. 2019, 287, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.H.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohydr. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Sinthusamran, S.; Kishimura, H. Gelatin from clown featherback skin: Extraction conditions. LWT-Food Sci. Technol. 2016, 66, 186–192. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Sabzipour, F.; Taghizadeh, M.S.; Moosavi-Nasab, M. Physicochemical, rheological, and molecular characterization of colloidal gelatin produced from Common carp by-products using microwave and ultrasound-assisted extraction. J. Texture Stud. 2019, 50, 416–425. [Google Scholar] [CrossRef]
- Klaiwong, T.; Hutangura, P.; Rutatip, S.; Wongsa-Ngasri, P.; Thumthanaruk, B. Comparative properties of pepsin hydrolyzed jellyfish protein from salted jellyfish. J. Agric. Sci. Technol. B 2014, 4, 555–564. [Google Scholar]
- Kaewruang, P.; Benjakul, S.; Prodpran, T.; Nalinanon, S. Physicochemical and functional properties of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as affected by extraction conditions. Food Biosci. 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Hong, Z.; Wen, H.; Hong, B.; Lin, R.; Chen, W.; Xie, Q.; Le, Q.; Yi, R.; Wu, H. Compositional and structural characteristics of pepsin-soluble type I collagen from the scales of red drum fish, Sciaenops ocellatus. Food Hydrocoll. 2022, 123, 107111. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.; Okda, H.; Amr, A. Preparation of poly (N-vinyl-2-pyrrolidone)/ammonium persulfate hydrogel embedded silver nanoparticles. Egypt. J. Chem. 2022, 65, 37–45. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and effects of crosslinking agent on cellulose-based hydrogels: A review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, G.; Gelli, R.; Mori, L.; Bonini, M. How to cross-link gelatin: The effect of glutaraldehyde and glyceraldehyde on the hydrogel properties. ACS Appl. Polym. Mater. 2023, 5, 9192–9202. [Google Scholar] [CrossRef]
- Ren, J.; Zhan, N.; Yang, J.; Xiang, X. Regulated the swelling properties of poly(acrylamide-co-acrylic acid) hydrogel by changing the charge density. Macromol. Chem. Phys. 2023, 224, 2300266. [Google Scholar] [CrossRef]
- Haryanto; Kim, S.; Kim, J.H.; Kim, J.O.; Ku, S.; Cho, H.; Han, D.H.; Huh, P. Fabrication of poly(ethylene oxide) hydrogels for wound dressing application using E-beam. Macromol. Res. 2014, 22, 131–138. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of water bound in hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, H.; Xiao, C. pH-sensitive cationic guar gum/poly (acrylic acid) polyelectrolyte hydrogels: Swelling and in vitro drug release. Carbohydr. Polym. 2007, 69, 774–783. [Google Scholar] [CrossRef]
- Charoenchokpanich, W.; Rungsardthong, V.; Vatanyoopaisarn, S.; Thumthanaruk, B.; Tamaki, Y. Salt reduction in salted jellyfish (Lobonema smithii) using a mechanical washing machine. Sci. Eng. Health Stud. 2020, 14, 184–192. [Google Scholar]
- Charoenchokpanich, W.; Muangrod, P.; Rungsardthong, V.; Vatanyoopaisarn, S.; Wonganu, B.; Roytrakul, S.; Thumthanaruk, B. Effect of hydrochloric acid extraction on yield and gel properties of gelatine from salted jellyfish by-products. E3S Web Conf. 2021, 302, 02009. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote) omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]







| Gelatin Sample | Gel Strength * (g) | Viscosity * (cP) | Gelling Temperature * (°C) | Melting Temperature * (°C) |
|---|---|---|---|---|
| BG | 846.31 ± 0.70 a | 30.06 ± 0.92 a | 22.22 ± 0.47 a | 30.23 ± 0.08 a |
| FG | 625.55 ± 0.40 b | 25.54 ± 0.02 b | 18.69 ± 0.05 b | 25.23 ± 0.02 b |
| WU24 | 460.02 ± 0.21 c | 24.45 ± 0.35 c | 12.70 ± 0.67 c | 22.48 ± 0.12 c |
| WO24 | 449.11 ± 0.86 d | 23.62 ± 0.55 d | 11.66 ± 0.85 c | 22.34 ± 1.02 c |
| WU48 | 412.63 ± 0.39 e | 22.44 ± 0.19 e | 9.93 ± 0.88 d | 20.11 ± 0.90 d |
| WO48 | 408.08 ± 0.52 f | 20.61 ± 0.33 f | 8.56 ± 0.20 e | 18.18 ± 0.64 e |
| Sample | Gel Fraction * (%) | Gel Porosity * (%) | |
|---|---|---|---|
| Bovine hydrogels (BGel) | BGel0.25 | 14.97 ± 0.46 c | 1.92 ± 0.09 b |
| BGel0.50 | 15.08 ± 0.82 c | 1.88 ± 0.05 bc | |
| BGel1.00 | 19.14 ± 0.60 a | 1.73 ± 0.01 d | |
| Fish hydrogels (FGel) | FGel0.25 | 13.02 ± 0.16 d | 2.15 ± 0.08 a |
| FGel0.50 | 16.27 ± 0.25 b | 1.90 ± 0.02 b | |
| FGel1.00 | 17.32 ± 0.59 b | 1.79 ± 0.04 cd | |
| Jellyfish hydrogels (JGel) | JGel0.25 | 12.58 ± 0.49 d | 2.20 ± 0.08 a |
| JGel0.50 | 14.30 ± 0.55 c | 1.91 ± 0.04 b | |
| JGel1.00 | 17.20 ± 0.97 b | 1.83 ± 0.05 bcd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenchokpanich, W.; Muangrod, P.; Roytrakul, S.; Rungsardthong, V.; Wonganu, B.; Charoenlappanit, S.; Casanova, F.; Thumthanaruk, B. Exploring the Model of Cefazolin Released from Jellyfish Gelatin-Based Hydrogels as Affected by Glutaraldehyde. Gels 2024, 10, 271. https://doi.org/10.3390/gels10040271
Charoenchokpanich W, Muangrod P, Roytrakul S, Rungsardthong V, Wonganu B, Charoenlappanit S, Casanova F, Thumthanaruk B. Exploring the Model of Cefazolin Released from Jellyfish Gelatin-Based Hydrogels as Affected by Glutaraldehyde. Gels. 2024; 10(4):271. https://doi.org/10.3390/gels10040271
Chicago/Turabian StyleCharoenchokpanich, Wiriya, Pratchaya Muangrod, Sittiruk Roytrakul, Vilai Rungsardthong, Benjamaporn Wonganu, Sawanya Charoenlappanit, Federico Casanova, and Benjawan Thumthanaruk. 2024. "Exploring the Model of Cefazolin Released from Jellyfish Gelatin-Based Hydrogels as Affected by Glutaraldehyde" Gels 10, no. 4: 271. https://doi.org/10.3390/gels10040271
APA StyleCharoenchokpanich, W., Muangrod, P., Roytrakul, S., Rungsardthong, V., Wonganu, B., Charoenlappanit, S., Casanova, F., & Thumthanaruk, B. (2024). Exploring the Model of Cefazolin Released from Jellyfish Gelatin-Based Hydrogels as Affected by Glutaraldehyde. Gels, 10(4), 271. https://doi.org/10.3390/gels10040271