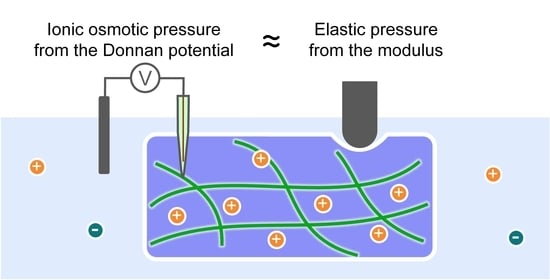
Experimental Verification of the Balance between Elastic Pressure and Ionic Osmotic Pressure of Highly Swollen Charged Gels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling Ratio and Modulus of the St-tetra-PEG Gels
2.2. Donnan Potential of the St-tetra-PEG Gels
2.3. Balance between Elasticity and Ionic Osmosis
2.4. St-tetra-PEG Gels in a Strong Ionic Environment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the St-tetra-PEG Gels
4.3. Indentation Test
4.4. Electric Potential Measurement
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breitenbach, J.W.; Karlinger, H. Über Quellung von vernetzter Polymethacrylsäure. Mon. Chem. 1949, 80, 312–313. [Google Scholar] [CrossRef]
- Katchalsky, A.; Michaeli, I. Polyelectrolyte gels in salt solutions. J. Polym. Sci. 1955, 15, 69–86. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase Transitions in Ionic Gels. Phys. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar] [CrossRef]
- Skouri, R.; Schosseler, F.; Munch, J.P.; Candau, S.J. Swelling and Elastic Properties of Polyelectrolyte Gels. Macromolecules 1995, 28, 197–210. [Google Scholar] [CrossRef]
- Horkay, F.; Tasaki, I.; Basser, P.J. Osmotic Swelling of Polyacrylate Hydrogels in Physiological Salt Solutions. Biomacromolecules 2000, 1, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Osada, Y.; Okuzaki, H.; Hori, H. A polymer gel with electrically driven motility. Nature 1992, 355, 242–244. [Google Scholar] [CrossRef]
- Ionov, L. Biomimetic hydrogel-based actuating systems. Adv. Funct. Mater. 2013, 23, 4555–4570. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Hong, W.; Zhao, X.; Zhou, J.; Suo, Z. A theory of coupled diffusion and large deformation in polymeric gels. J. Mech. Phys. Solids 2008, 56, 1779–1793. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Maroto-Centeno, J.A.; Forcada, J.; Hidalgo-Alvarez, R. Gel swelling theories: The classical formalism and recent approaches. Soft Matter 2011, 7, 10536–10547. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Rička, J.; Tanaka, T. Swelling of Ionic Gels: Quantitative Performance of the Donnan Theory. Macromolecules 1984, 17, 2916–2921. [Google Scholar] [CrossRef]
- Morozova, S.; Muthukumar, M. Elasticity at Swelling Equilibrium of Ultrasoft Polyelectrolyte Gels: Comparisons of Theory and Experiments. Macromolecules 2017, 50, 2456–2466. [Google Scholar] [CrossRef]
- Barenbrug, T.M.A.O.M.; Smit, J.A.M.; Bedeaux, D. Highly swollen gels of semi-flexible polyelectrolyte chains near the rod limit. Polym. Gels Netw. 1995, 3, 331–373. [Google Scholar] [CrossRef]
- Treloar, L.R.G. The Physics of Rubber Elasticity; Clarendon Press: Oxford, UK, 1976. [Google Scholar]
- Nakajima, T.; Chida, T.; Mito, K.; Kurokawa, T.; Gong, J.P. Double-network gels as polyelectrolyte gels with salt-insensitive swelling properties. Soft Matter 2020, 16, 5487–5496. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Katashima, T.; Li, X.; Mitsukami, Y.; Yokoyama, Y.; Sakumichi, N.; Chung, U.-I.; Shibayama, M.; Sakai, T. Swelling Behaviors of Hydrogels with Alternating Neutral/Highly Charged Sequences. Macromolecules 2020, 53, 8244–8254. [Google Scholar] [CrossRef]
- Katchalsky, A.; Lifson, S.; Exsenberg, H. Equation of swelling for polyelectrolyte gels. J. Polym. Sci. 1951, 7, 571–574. [Google Scholar] [CrossRef]
- Hasa, J.; Ilavský, M.; Dušek, K. Deformational, swelling, and potentiometric behavior of ionized poly(methacrylic acid) gels. I. Theory. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 253–262. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H.; Dobrynin, A.V.; Joanny, J.-F. Elastic modulus and equilibrium swelling of polyelectrolyte gels. Macromolecules 1996, 29, 398–406. [Google Scholar] [CrossRef]
- Vamvakaki, M.; Patrickios, C.S. Polyelectrolytic Amphiphilic Model Networks in Water: A Molecular Thermodynamic Theory for Their Microphase Separation. J. Phys. Chem. B 2001, 105, 4979–4986. [Google Scholar] [CrossRef]
- Hoshino, K.-i.; Nakajima, T.; Matsuda, T.; Sakai, T.; Gong, J.P. Network elasticity of a model hydrogel as a function of swelling ratio: From shrinking to extreme swelling states. Soft Matter 2018, 14, 9693–9701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisato, G.; Skouri, R.; Schosseler, F.; Munch, J.; Candau, S.J. Elastic behaviour of salt-free polyelectrolyte gels. Faraday Discuss. 1995, 101, 133–146. [Google Scholar] [CrossRef]
- Barrat, J.-L.; Joanny, J.-F.; Pincus, P. On the scattering properties of polyelectrolyte gels. J. Phys. II 1992, 2, 1531–1544. [Google Scholar] [CrossRef]
- Prudnikova, K.; Utz, M. Electromechanical equilibrium properties of poly(acrylic acid/acrylamide) hydrogels. Macromolecules 2012, 45, 1041–1045. [Google Scholar] [CrossRef]
- Manning, G.S. Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions I. Colligative Properties. J. Chem. Phys. 1969, 51, 924–933. [Google Scholar] [CrossRef]
- Safronov, A.P.; Shklyar, T.F.; Borodin, V.S.; Smirnova, Y.A.; Sokolov, S.Y.; Pollack, G.H.; Blyakhman, F.A. Donnan Potential in Hydrogels of Poly(Methacrylic Acid) and its Potassium Salt. In Water and the Cell; Springer: Dordrecht, The Netherlands, 2006; pp. 273–284. ISBN 9781626239777. [Google Scholar]
- Guo, H.; Kurokawa, T.; Takahata, M.; Hong, W.; Katsuyama, Y.; Luo, F.; Ahmed, J.; Nakajima, T.; Nonoyama, T.; Gong, J.P. Quantitative Observation of Electric Potential Distribution of Brittle Polyelectrolyte Hydrogels Using Microelectrode Technique. Macromolecules 2016, 49, 3100–3108. [Google Scholar] [CrossRef]
- Shibayama, M. Spatial inhomogeneity and dynamic fluctuations of polymer gels. Macromol. Chem. Phys. 1998, 199, 1–30. [Google Scholar] [CrossRef]
- Seiffert, S. Origin of nanostructural inhomogeneity in polymer-network gels. Polym. Chem. 2017, 8, 4472–4487. [Google Scholar] [CrossRef]
- Okay, O.; Durmaz, S. Charge density dependence of elastic modulus of strong polyelectrolyte hydrogels. Polymer 2002, 43, 1215–1221. [Google Scholar] [CrossRef]
- Li, J.; Suo, Z.; Vlassak, J.J. A model of ideal elastomeric gels for polyelectrolyte gels. Soft Matter 2014, 10, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.-I. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Sakai, T. Experimental verification of homogeneity in polymer gels. Polym. J. 2014, 46, 517–523. [Google Scholar] [CrossRef]
- Akagi, Y.; Katashima, T.; Katsumoto, Y.; Fujii, K.; Matsunaga, T.; Chung, U.-I.; Shibayama, M.; Sakai, T. Examination of the theories of rubber elasticity using an ideal polymer network. Macromolecules 2011, 44, 5817–5821. [Google Scholar] [CrossRef]
- Lange, F.; Schwenke, K.; Kurakazu, M.; Akagi, Y.; Chung, U.-I.; Lang, M.; Sommer, J.U.; Sakai, T.; Saalwächter, K. Connectivity and structural defects in model hydrogels: A combined proton NMR and Monte Carlo simulation study. Macromolecules 2011, 44, 9666–9674. [Google Scholar] [CrossRef]
- Akagi, Y.; Gong, J.P.; Chung, U.-I.; Sakai, T. Transition between phantom and affine network model observed in polymer gels with controlled network structure. Macromolecules 2013, 46, 1035–1040. [Google Scholar] [CrossRef]
- Akagi, Y.; Katashima, T.; Sakurai, H.; Chung, U.-I.; Sakai, T. Ultimate elongation of polymer gels with controlled network structure. RSC Adv. 2013, 3, 13251–13258. [Google Scholar] [CrossRef]
- Nakajima, T.; Sato, H.; Zhao, Y.; Kawahara, S.; Kurokawa, T.; Sugahara, K.; Gong, J.P. A universal molecular stent method to toughen any hydrogels based on double network concept. Adv. Funct. Mater. 2012, 22, 4426–4432. [Google Scholar] [CrossRef]
- Nakajima, T.; Fukuda, Y.; Kurokawa, T.; Sakai, T.; Chung, U.-I.; Gong, J.P. Synthesis and fracture process analysis of double network hydrogels with a well-defined first network. ACS Macro Lett. 2013, 2, 518–521. [Google Scholar] [CrossRef]
- Konda, A.; Urayama, K.; Takigawa, T. Strain-rate-dependent poisson’s ratio and stress of polymer gels in solvents revealed by ultraslow stretching. Macromolecules 2011, 44, 3000–3006. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, X.; Vlassak, J.J.; Suo, Z. Using indentation to characterize the poroelasticity of gels. Appl. Phys. Lett. 2010, 96, 121904. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, T.; Hoshino, K.-i.; Guo, H.; Kurokawa, T.; Gong, J.P. Experimental Verification of the Balance between Elastic Pressure and Ionic Osmotic Pressure of Highly Swollen Charged Gels. Gels 2021, 7, 39. https://doi.org/10.3390/gels7020039
Nakajima T, Hoshino K-i, Guo H, Kurokawa T, Gong JP. Experimental Verification of the Balance between Elastic Pressure and Ionic Osmotic Pressure of Highly Swollen Charged Gels. Gels. 2021; 7(2):39. https://doi.org/10.3390/gels7020039
Chicago/Turabian StyleNakajima, Tasuku, Ken-ichi Hoshino, Honglei Guo, Takayuki Kurokawa, and Jian Ping Gong. 2021. "Experimental Verification of the Balance between Elastic Pressure and Ionic Osmotic Pressure of Highly Swollen Charged Gels" Gels 7, no. 2: 39. https://doi.org/10.3390/gels7020039
APA StyleNakajima, T., Hoshino, K. -i., Guo, H., Kurokawa, T., & Gong, J. P. (2021). Experimental Verification of the Balance between Elastic Pressure and Ionic Osmotic Pressure of Highly Swollen Charged Gels. Gels, 7(2), 39. https://doi.org/10.3390/gels7020039