Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications
Abstract
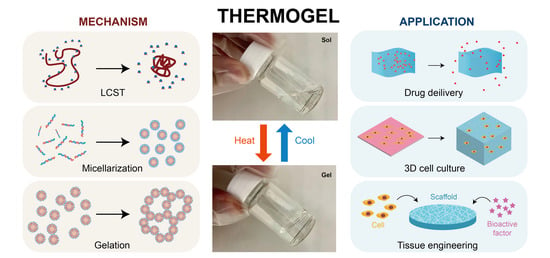
:1. Introduction
2. Thermogelling Mechanism/Thermogelling Properties
2.1. LCST
2.2. Polymer Configurations and Micelle Properties
2.3. Gelling Mechanism
3. Biomedical Applications
3.1. Drug Delivery
3.2. Three-Dimensional Cell/Stem Cell Culture
3.3. Tissue Engineering
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Xue, K.; Wang, X.; Yong, P.W.; Young, D.J.; Wu, Y.-L.; Li, Z.; Loh, X.J. Hydrogels as Emerging Materials for Translational Biomedicine. Adv. Ther. 2019, 2, 1800088. [Google Scholar] [CrossRef] [Green Version]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Loh, X.J.; Scherman, O.A. Polymeric and Self Assembled Hydrogels: From Fundamental Understanding to Applications; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 11. [Google Scholar]
- Xue, K.; Liow, S.S.; Karim, A.A.; Li, Z.; Loh, X.J. A Recent Perspective on Noncovalently Formed Polymeric Hydrogels. Chem. Rec. 2018, 18, 1517–1529. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.C.; Lin, Q.; Xue, K.; Loh, X.J. Recent advances in supramolecular hydrogels for biomedical applications. Mater. Today Adv. 2019, 3, 100021. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Noth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Dou, Q.Q.; Liow, S.S.; Ye, E.; Lakshminarayanan, R.; Loh, X.J. Biodegradable thermogelling polymers: Working towards clinical applications. Adv. Healthc. Mater. 2014, 3, 977–988. [Google Scholar] [CrossRef]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Ko, D.Y.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef]
- Akash, M.S.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.C.; Lai, J.Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.Y.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar] [CrossRef]
- Patel, M.; Lee, H.J.; Park, S.; Kim, Y.; Jeong, B. Injectable thermogel for 3D culture of stem cells. Biomaterials 2018, 159, 91–107. [Google Scholar] [CrossRef]
- Chee, P.L.; Young, D.J.; Loh, X.J. Chapter 7. Degradation Behaviour of Biodegradable Thermogels. In Biodegradable Thermogels; RSC Publishing: London, UK, 2018; pp. 113–132. [Google Scholar] [CrossRef]
- Larrañeta, E.; Isasi, J.R. Non-covalent hydrogels of cyclodextrins and poloxamines for the controlled release of proteins. Carbohydr. Polym. 2014, 102, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Supramolecular self-healing materials from non-covalent cross-linking host–guest interactions. Chem. Commun. 2020, 56, 4381–4395. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-Ionic Thermoresponsive Polymers in Water; Springer: Berlin/Heidelberg, Germany, 2010; Volume 242, pp. 29–89. [Google Scholar]
- Hammouda, B.; Ho, D.; Kline, S. SANS from Poly(ethylene oxide)/Water Systems. Macromolecules 2002, 35, 8578–8585. [Google Scholar] [CrossRef]
- Schild, H.G.; Tirrell, D.A. Microcalorimetric detection of lower critical solution temperatures in aqueous polymer solutions. J. Phys. Chem. 1990, 94, 4352–4356. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Crespy, D.; Rossi, R.M. Temperature—Responsive polymers with LCST in the physiological range and their applications in textiles. Polym. Int. 2007, 56, 1461–1468. [Google Scholar] [CrossRef]
- Swier, S.; Van Durme, K.; Van Mele, B. Modulated-temperature differential scanning calorimetry study of temperature-induced mixing and demixing in poly (vinylmethylether)/water. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1824–1836. [Google Scholar] [CrossRef]
- Schäfer-Soenen, H.; Moerkerke, R.; Berghmans, H.; Koningsveld, R.; Dušek, K.; Šolc, K. Zero and Off-Zero Critical Concentrations in Systems Containing Polydisperse Polymers with Very High Molar Masses. 2. The System Water−Poly(vinyl methyl ether). Macromolecules 1997, 30, 410–416. [Google Scholar] [CrossRef]
- Aseyev, V.O.; Tenhu, H.; Winnik, F.M. Temperature dependence of the colloidal stability of neutral amphiphilic polymers in water. In Conformation-Dependent Design of Sequences in Copolymers II; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–85. [Google Scholar]
- Tager, A.; Safronov, A.; Berezyuk, E.; Galaev, I.Y. Lower critical solution temperature and hydrophobic hydration in aqueous polymer solutions. Colloid Polym. Sci. 1994, 272, 1234–1239. [Google Scholar] [CrossRef]
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Edit. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hayashi, T.; Hikiri, S.; Ikeguchi, M.; Kinoshita, M. Mechanism of globule-to-coil transition of poly(N-isopropylacrylamide) in water: Relevance to cold denaturation of a protein. J. Mol. Liq. 2019, 292. [Google Scholar] [CrossRef]
- Oliveira, T.E.d.; Mukherji, D.; Kremer, K.; Netz, P.A. Effects of stereochemistry and copolymerization on the LCST of PNIPAm. J. Chem. Phys. 2017, 146, 034904. [Google Scholar] [CrossRef]
- Polak, J.; Ondo, D.; Heyda, J. Thermodynamics of N-Isopropylacrylamide in Water: Insight from Experiments, Simulations, and Kirkwood-Buff Analysis Teamwork. J. Phys. Chem. B 2020, 124, 2495–2504. [Google Scholar] [CrossRef]
- Custodio, K.K.S.; Claudio, G.C.; Nellas, R.B. Structural Dynamics of Neighboring Water Molecules of N-Isopropylacrylamide Pentamer. ACS Omega 2020, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Barbier, L.; Li, Z.; Ji, X.; Le Blay, H.; Liu, J.; Lam, J.W.; Marcellan, A.; Tang, B.Z. Making Hydrogels Stronger through Hydrophilicity-Hydrophobicity Transformation, Thermoresponsive Morphomechanics and Crack Multifurcation. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Therien-Aubin, H.; Wu, Z.L.; Nie, Z.; Kumacheva, E. Multiple shape transformations of composite hydrogel sheets. J. Am. Chem. Soc. 2013, 135, 4834–4839. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.A.; Maikawa, C.L.; Lopez Hernandez, H.; Appel, E.A. Controlling properties of thermogels by tuning critical solution behaviour of ternary copolymers. Polym. Chem. 2021, 12, 1918–1923. [Google Scholar] [CrossRef]
- Bayat, N.; Zhang, Y.; Falabella, P.; Menefee, R.; Whalen, R.J.J.; Humayun, M.S.; Thompson, M.E. A reversible thermoresponsive sealant for temporary closure of ocular trauma. Sci. Transl. Med. 2017, 9, eaan3879. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Chou, P.Y.; Chen, S.H.; Chen, C.H.; Chen, S.H.; Fong, Y.T.; Chen, J.P. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater. 2017, 63, 85–95. [Google Scholar] [CrossRef]
- Luo, Z.; Xue, K.; Zhang, X.; Lim, J.Y.C.; Lai, X.; Young, D.J.; Zhang, Z.-X.; Wu, Y.-L.; Loh, X.J. Thermogelling chitosan-based polymers for the treatment of oral mucosa ulcers. Biomater. Sci. 2020, 8, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.W.; Wang, Y.; Hu, X.Z.; Gong, H.N.; Li, R.H.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide-PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Lee, H.T.; Shim, W.S.; Park, I.; Lee, H.; Chang, T.; Kim, S.W.; Lee, D.S. Poly (D, L-lactic acid-co-glycolic acid)-b-poly (ethylene glycol)-b-poly (D, L-lactic acid-co-glycolic acid) triblock copolymer and thermoreversible phase transition in water. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 61, 188–196. [Google Scholar] [CrossRef]
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 2016, 50, 3–22. [Google Scholar] [CrossRef]
- Badi, N.; Lutz, J.F. PEG-based thermogels: Applicability in physiological media. J. Control. Release 2009, 140, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Dong, W.; Tian, Y. Temperature-Responsive Phase Transition of Polymer Vesicles: Real-Time Morphology Observation and Molecular Mechanism. J. Phys. Chem. B 2007, 111, 1262–1270. [Google Scholar] [CrossRef]
- Li, Z.; Li, J. Control of hyperbranched structure of polycaprolactone/poly (ethylene glycol) polyurethane block copolymers by glycerol and their hydrogels for potential cell delivery. J. Phys. Chem. B 2013, 117, 14763–14774. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Liu, K.L.; Ni, X.; Li, J. Biodegradable hyperbranched amphiphilic polyurethane multiblock copolymers consisting of poly(propylene glycol), poly(ethylene glycol), and polycaprolactone as in situ thermogels. Biomacromolecules 2012, 13, 3977–3989. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Chee, C.P.T.; Loh, X.J. Supramolecular thermogels from branched PCL-containing polyurethanes. RSC Adv. 2020, 10, 39109–39120. [Google Scholar] [CrossRef]
- Arranja, A.; Waton, G.; Schosseler, F.; Mendes, E. Lack of a unique kinetic pathway in the growth and decay of Pluronic micelles. Soft Matter 2016, 12, 769–778. [Google Scholar] [CrossRef]
- Landazuri, G.; Fernandez, V.V.; Soltero, J.F.; Rharbi, Y. Kinetics of the sphere-to-rod like micelle transition in a pluronic triblock copolymer. J. Phys. Chem. B 2012, 116, 11720–11727. [Google Scholar] [CrossRef]
- Puig-Rigall, J.; Obregon-Gomez, I.; Monreal-Pérez, P.; Radulescu, A.; Blanco-Prieto, M.J.; Dreiss, C.A.; González-Gaitano, G. Phase behaviour, micellar structure and linear rheology of tetrablock copolymer Tetronic 908. J. Colloid Interface Sci. 2018, 524, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Lu, J.; Bates, F.S.; Lodge, T.P. Remarkable Effect of Molecular Architecture on Chain Exchange in Triblock Copolymer Micelles. Macromolecules 2015, 48, 2667–2676. [Google Scholar] [CrossRef]
- Zinn, T.; Willner, L.; Pipich, V.; Richter, D.; Lund, R. Molecular Exchange Kinetics of Micelles: Corona Chain Length Dependence. ACS Macro Lett. 2016, 5, 884–888. [Google Scholar] [CrossRef]
- Lim, H.K.; Young, D.J.; Loh, X.J. Chapter 4. Biodegradable Thermogelling Polymers for Drug Delivery. In Biodegradable Thermogels; RSC Publishing: London, UK, 2018; pp. 76–86. [Google Scholar] [CrossRef]
- O’Lenick, T.G.; Jin, N.; Woodcock, J.W.; Zhao, B. Rheological properties of aqueous micellar gels of a thermo- and pH-sensitive ABA triblock copolymer. J. Phys. Chem. B 2011, 115, 2870–2881. [Google Scholar] [CrossRef]
- Jiang, X.; Ge, Z.; Xu, J.; Liu, H.; Liu, S. Fabrication of Multiresponsive Shell Cross-Linked Micelles Possessing pH-Controllable Core Swellability and Thermo-Tunable Corona Permeability. Biomacromolecules 2007, 8, 3184–3192. [Google Scholar] [CrossRef]
- Loh, X.J.; Zhang, Z.-X.; Wu, Y.-L.; Lee, T.S.; Li, J. Synthesis of Novel Biodegradable Thermoresponsive Triblock Copolymers Based on Poly[(R)-3-hydroxybutyrate] and Poly(N-isopropylacrylamide) and Their Formation of Thermoresponsive Micelles. Macromolecules 2009, 42, 194–202. [Google Scholar] [CrossRef]
- Chen, L.; Ci, T.; Yu, L.; Ding, J. Effects of Molecular Weight and Its Distribution of PEG Block on Micellization and Thermogellability of PLGA–PEG–PLGA Copolymer Aqueous Solutions. Macromolecules 2015, 48, 3662–3671. [Google Scholar] [CrossRef]
- Huang, P.; Song, H.; Zhang, Y.; Liu, J.; Cheng, Z.; Liang, X.-J.; Wang, W.; Kong, D.; Liu, J. FRET-enabled monitoring of the thermosensitive nanoscale assembly of polymeric micelles into macroscale hydrogel and sequential cognate micelles release. Biomaterials 2017, 145, 81–91. [Google Scholar] [CrossRef]
- Cui, S.; Yu, L.; Ding, J. Semi-bald Micelles and Corresponding Percolated Micelle Networks of Thermogels. Macromolecules 2018, 51, 6405–6420. [Google Scholar] [CrossRef]
- Cui, S.; Yu, L.; Ding, J. Thermogelling of Amphiphilic Block Copolymers in Water: ABA Type versus AB or BAB Type. Macromolecules 2019, 52, 3697–3715. [Google Scholar] [CrossRef]
- Li, H.; Ji, Q.X.; Chen, X.M.; Sun, Y.; Xu, Q.C.; Deng, P.P.; Hu, F.; Yang, J.J. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J. Biomed. Mater. Res. Part A 2017, 105, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Censi, R.; Vermonden, T.; van Steenbergen, M.J.; Deschout, H.; Braeckmans, K.; De Smedt, S.C.; van Nostrum, C.F.; di Martino, P.; Hennink, W.E. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control Release 2009, 140, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sabharwal, V.; Shlykova, N.; Okonkwo, O.S.; Pelton, S.I.; Kohane, D.S. Treatment of Streptococcus pneumoniae otitis media in a chinchilla model by transtympanic delivery of antibiotics. JCI Insight 2018, 3, e123415. [Google Scholar] [CrossRef]
- Kim, Y.C.; Shin, M.D.; Hackett, S.F.; Hsueh, H.T.; e Silva, R.L.; Date, A.; Han, H.; Kim, B.-J.; Xiao, A.; Kim, Y. Gelling hypotonic polymer solution for extended topical drug delivery to the eye. Nat. Biomed. Eng. 2020, 4, 1053–1062. [Google Scholar] [CrossRef]
- Stearne, M.R.; Palmer, S.L.; Hammersley, M.S.; Franklin, S.L.; Spivey, R.S.; Levy, J.C.; Tidy, C.R.; Bell, N.J.; Steemson, J.; Barrow, B.A.; et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ Br. Med. J. 1998, 317, 703–713. [Google Scholar]
- Chen, Y.; Li, Y.; Shen, W.; Li, K.; Yu, L.; Chen, Q.; Ding, J. Controlled release of liraglutide using thermogelling polymers in treatment of diabetes. Sci. Rep. 2016, 6, 31593. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.-J.; Lee, Y.-J.; Jeon, H.-Y.; Kim, M.; Han, E.-T.; Park, W.S.; Hong, S.-H.; Kim, Y.-M.; Ha, K.-S. Application of elastin-like biopolymer-conjugated C-peptide hydrogel for systemic long-term delivery against diabetic aortic dysfunction. Acta Biomater. 2020, 118, 32–43. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Thambi, T.; Gil, M.S.; Lee, D.S. Temperature and pH-sensitive injectable hydrogels based on poly(sulfamethazine carbonate urethane) for sustained delivery of cationic proteins. Polymer 2017, 109, 38–48. [Google Scholar] [CrossRef]
- Pacelli, S.; Acosta, F.; Chakravarti, A.R.; Samanta, S.G.; Whitlow, J.; Modaresi, S.; Ahmed, R.P.H.; Rajasingh, J.; Paul, A. Nanodiamond-based injectable hydrogel for sustained growth factor release: Preparation, characterization and in vitro analysis. Acta Biomater. 2017, 58, 479–491. [Google Scholar] [CrossRef]
- Xue, K.; Zhao, X.X.; Zhang, Z.X.; Qiu, B.Y.; Tan, Q.S.W.; Ong, K.H.; Liu, Z.P.; Parikh, B.H.; Barathi, V.A.; Yu, W.M.; et al. Sustained delivery of anti-VEGFs from thermogel depots inhibits angiogenesis without the need for multiple injections. Biomater. Sci. 2019, 7, 4603–4614. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Chen, X.; Cao, F.; Guo, W.; Tang, J.; Cai, C.; Cui, S.; Yang, X.; Yu, L.; Su, Y. An Intelligent Transdermal Formulation of ALA-Loaded Copolymer Thermogel with Spontaneous Asymmetry by Using Temperature-Induced Sol–Gel Transition and Gel–Sol (Suspension) Transition on Different Sides. Adv. Funct. Mater. 2021, 31, 2100349. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Zhang, Y.-C.; Huang, X.-H.; Xie, Y.-Q.; Xiang, Y. A metabolomic study of rats with doxorubicin-induced cardiomyopathy and Shengmai injection treatment. PLoS ONE 2015, 10, e0125209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and its formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) That Releases Doxorubicin-Encapsulated Micelles as a Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Wu, Y.L.; Wang, H.; Qiu, Y.K.; Liow, S.S.; Li, Z.; Loh, X.J. PHB-Based Gels as Delivery Agents of Chemotherapeutics for the Effective Shrinkage of Tumors. Adv. Healthc. Mater. 2016, 5, 2679–2685. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Shao, J.D.; Ruan, C.S.; Xie, H.H.; Li, Z.B.; Wang, H.Y.; Chu, P.K.; Yu, X.F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Young, D.J.; Loh, X.J. From Bench to Bedside–OncoGel™, an In Situ Hydrogel for In Vivo Applications. Biodegrad. Thermogels 2018, 2, 133. [Google Scholar]
- Choi, B.G.; Park, M.H.; Cho, S.H.; Joo, M.K.; Oh, H.J.; Kim, E.H.; Park, K.; Han, D.K.; Jeong, B. In situ thermal gelling polypeptide for chondrocytes 3D culture. Biomaterials 2010, 31, 9266–9272. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Li, H.; Yang, C.; Cai, X. Study of differential properties of fibrochondrocytes and hyaline chondrocytes in growing rabbits. Br. J. Oral Maxillofac. Surg. 2015, 53, 187–193. [Google Scholar] [CrossRef]
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.Y.; Patel, M.; Lee, H.J.; Jeong, B. Coordinating Thermogel for Stem Cell Spheroids and Their Cyto-Effectiveness. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, H.J.; Jeong, B. Injectable Polypeptide Thermogel as a Tissue Engineering System for Hepatogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 11568–11576. [Google Scholar] [CrossRef]
- Lee, S.S.; Choi, G.E.; Lee, H.J.; Kim, Y.; Choy, J.H.; Jeong, B. Layered Double Hydroxide and Polypeptide Thermogel Nanocomposite System for Chondrogenic Differentiation of Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 42668–42675. [Google Scholar] [CrossRef]
- Patel, M.; Moon, H.J.; Du, Y.K.; Jeong, B. Composite System of Graphene Oxide and Polypeptide Thermogel As an InjecTable 3D Scaffold for Adipogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 5160–5169. [Google Scholar] [CrossRef]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef] [PubMed]
- Baei, P.; Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 131–141. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, Z.; Niu, H.; Gao, N.; Guan, Y.; Li, C.; Dang, Y.; Cui, X.; Liu, X.L.; Duan, Y.; et al. An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Sci. Rep. 2018, 8, 1371. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J.; et al. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Jiang, X.; Lin, Q.; Xu, H.L.; Huang, Y.D.; Lu, C.T.; Cai, J. Thermosensitive heparin-poloxamer hydrogels enhance the effects of GDNF on neuronal circuit remodeling and neuroprotection after spinal cord injury. J. Biomed. Mater. Res. A 2017, 105, 2816–2829. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Lin, R.; Yun, S.; Du, Y.; Wang, L.; Yao, Q.; Zannettino, A.; Zhang, H. Allogeneic primary mesenchymal stem/stromal cell aggregates within poly(N-isopropylacrylamide-co-acrylic acid) hydrogel for osteochondral regeneration. Appl. Mater. Today 2020, 18, 100487. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chang, F.; Xu, W.; Ding, J. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 79–87. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.J.; Zhao, X.R.; Zhu, Y.F.; Yu, J.K. 3D-Printed Poly(epsilon-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef]
- Schulz, A.; Wahl, S.; Rickmann, A.; Ludwig, J.; Stanzel, B.V.; von Briesen, H.; Szurman, P. Age-Related Loss of Human Vitreal Viscoelasticity. Transl. Vis. Sci. Technol. 2019, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Su, X.; Loh, X.J. Polymeric hydrogels as a vitreous replacement strategy in the eye. Biomaterials 2021, 268, 120547. [Google Scholar] [CrossRef]
- Liu, Z.; Liow, S.S.; Lai, S.L.; Alli-Shaik, A.; Holder, G.E.; Parikh, B.H.; Krishnakumar, S.; Li, Z.; Tan, M.J.; Gunaratne, J.; et al. Retinal-detachment repair and vitreous-like-body reformation via a thermogelling polymer endotamponade. Nat. Biomed. Eng. 2019, 3, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Okamoto, F.; Hoshi, S.; Katashima, T.; Zujur, D.C.; Li, X.; Shibayama, M.; Gilbert, E.P.; Chung, U.-i.; Ohba, S.; et al. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat. Biomed. Eng. 2017, 1, 0044. [Google Scholar] [CrossRef]
- Xue, K.; Liu, Z.; Lin, Q.; Lim, J.Y.C.; Tang, K.Y.; Wong, S.L.; Parikh, B.H.; Su, X.; Loh, X.J. PCL-Based Thermogelling Polymer: Molecular Weight Effects on Its Suitability as Vitreous Tamponade. ACS Appl. Bio Mater. 2020, 3, 9043–9053. [Google Scholar] [CrossRef]
- Xue, K.; Liu, Z.; Jiang, L.; Kai, D.; Li, Z.; Su, X.; Loh, X.J. A new highly transparent injectable PHA-based thermogelling vitreous substitute. Biomater. Sci. 2020, 8, 926–936. [Google Scholar] [CrossRef] [PubMed]






| Polymer | Abbreviation | LCST (°C) | Reference |
|---|---|---|---|
| Poly(ethylene glycol) | PEG/PEO | 106~115 | [25] |
| Poly(propylene glycol) | PPG/PPO | 10~40 | [26] |
| Poly(vinylalcohol) | PVA/PVAl | 125 | [27] |
| Poly(N-isopropylacrylamide) | PNIPAM/PNIPAAM | 32 | [28] |
| Poly(methyl vinyl ether) | PMVE | 28~34 | [29,30] |
| Poly(N-vinyl caprolactam) | PNVCa/PVCL | 30~50 | [31,32] |
| Polymer Name | Drug | Application | Reference |
|---|---|---|---|
| Pluronic F-127 | Brimonidine tartrate (BT), brinzolamide (BRZ), cyclosporine | Eye drop | [71] |
| PCGA-PEG-PCGA | Liraglutide | Treat type 2 diabetes | [73] |
| Elastin-like thermogel | Human C-peptide | Treat diabetic complications | [74] |
| PEG- PSMCU | lysozyme | In situ protein delivery | [75] |
| ND-based gelatin chitosan | VEGF | Growth factor bases therapies | [76] |
| PCL-based polyurethane thermogel | Anti-VEGF | Treat proliferative vascular diseases | [77] |
| PLGA-PEG-PLGA | ALA | Transdermal drug delivery | [78] |
| Alginate-g-PNIPAM | DOX | Treat cancer | [81] |
| PHB-based polyester | DOX, PTX | Treat Hepatocellular carcinoma | [82] |
| PDLLA-PEG-PDLLA | BP | Photothermal therapy | [84] |
| Base Thermogel | Additional Functionalities | Polymer Effect | Application | Reference |
|---|---|---|---|---|
| Chitosan | GNPs | Electro-conductive | Cardiac repair | [94] |
| PNIPAM | PLGA, PVP/H2O2 | Release oxygen | Cardiac repair | [95] |
| Poloxamer | Heparin | Release bFGF, NGF | Neurilemmal cells regeneration | [96] |
| Poloxamer | Heparin | Release GDNF | Spinal cord injury | [97] |
| P(NIPAAM-AA) | BMSCs | Scaffold for BMSCs | Osteochondral regeneration | [99] |
| PLGA-PEG-PLGA | BMSCs | Scaffold for BMSCs | Full-thickness cartilage repair | [100] |
| Chitosan- β-glycerophosphate | PCL | Scaffold for BMSCs | Bone tissue engineering | [101] |
| PCL-based thermogel | None | Vitreous tamponade | Retinal detachment | [106] |
| PHA-based thermogel | None | Vitreous tamponade | Retinal detachment | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. https://doi.org/10.3390/gels7030077
Zhang K, Xue K, Loh XJ. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels. 2021; 7(3):77. https://doi.org/10.3390/gels7030077
Chicago/Turabian StyleZhang, Kaiwen, Kun Xue, and Xian Jun Loh. 2021. "Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications" Gels 7, no. 3: 77. https://doi.org/10.3390/gels7030077
APA StyleZhang, K., Xue, K., & Loh, X. J. (2021). Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels, 7(3), 77. https://doi.org/10.3390/gels7030077