Cellulose-Based Hydrogels and Aerogels Embedded with Silver Nanoparticles: Preparation and Characterization
Abstract
:1. Introduction
2. Results and Discussion
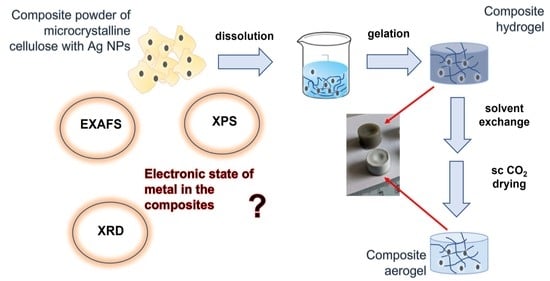
2.1. Preparation, Morphology, and Porous Structure of the Composites
2.2. Characterization of the Composites with Synchrotron-Based Techniques
2.3. Surface Analysis of the Composites
2.4. Thermal Behavior and Stability of the Composites
3. Conclusions
4. Experimental Part Materials and Methods
4.1. Materials
4.2. Preparation of Ag-MCC Composite
4.3. Preparation of Ag-AC Composite
4.4. Characterization
4.4.1. Synchrotron Radiation-Based Techniques
4.4.2. X-ray Photoelectron Spectroscopy (XPS)
4.4.3. Porosity, Microstructure, and Morphology
4.4.4. Thermal Stability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Pryakhina, T.A.; Vasil’ev, V.G.; Gallyamov, M.O.; Muzafarov, A.M. A study of the hydrosilylation approach to a one-pot synthesis of silicone aerogels in supercritical CO2. J. Supercrit. Fluids 2018, 133, 512–518. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Stakhanov, A.I.; Zefirov, V.V.; Pavlov, A.A.; Lokshin, B.V.; Gallyamov, M.O. Thermal oxidation of polypropylene catalyzed by manganese oxide aerogel in oxygen-enriched supercritical carbon dioxide. J. Supercrit. Fluids 2020, 158, 104744. [Google Scholar] [CrossRef]
- Budtova, T.; Navard, P. Cellulose in NaOH—Water based solvents: A review. Cellulose 2015, 23, 5–55. [Google Scholar] [CrossRef] [Green Version]
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.M.; Lichtenegger, H.C.; Rosenau, T.; Liebner, F. Impact of selected solvent systems on the pore and solid structure of cellulose aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef] [Green Version]
- Gavillon, R.; Budtova, T. Aerocellulose: New highly porous cellulose prepared from cellulose-NaOH aqueous solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from cellulose-ionic liquid solutions: Preparation, properties and comparison with cellulose-NaOH and cellulose-NMMO routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Takeshita, S.; Zhao, S.; Malfait, W.J. Transparent, Aldehyde-Free Chitosan Aerogel. Carbohydr. Polym. 2021, 251, 117089. [Google Scholar] [CrossRef]
- Gurikov, P.; Raman, S.P.; Weinrich, D.; Fricke, M.; Smirnova, I. A novel approach to alginate aerogels: Carbon dioxide induced gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar] [CrossRef]
- Dogenski, M.; Gurikov, P.; Baudron, V.; de Oliveira, J.V.; Smirnova, I.; Ferreira, S.R.S. Starch-based aerogels obtained via solvent-induced gelation. Gels 2020, 6, 32. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, G.C.; Learmonth, D.A.; Vallejo, M.C.; Davila, S.P.; González, P.; Sousa, R.A.; Oliveira, A.L. Supercritical CO2 technology: The next standard sterilization technique? Mater. Sci. Eng. C 2019, 99, 520–540. [Google Scholar] [CrossRef]
- Lopes, J.M.; Mustapa, A.N.; Pantić, M.; Bermejo, M.D.; Martín, Á.; Novak, Z.; Knez, Ž.; Cocero, M.J. Preparation of cellulose aerogels from ionic liquid solutions for supercritical impregnation of phytol. J. Supercrit. Fluids 2017, 130, 17–22. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New Horizons in Hydrogels for Methotrexate Delivery. Gels 2021, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, R.; Jonoobi, M.; Amirabad, L.M.; Oksman, K.; Taheri, A.R. Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. Int. J. Biol. Macromol. 2019, 136, 796–803. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Demilecamps, A.; Beauger, C.; Hildenbrand, C.; Rigacci, A.; Budtova, T. Cellulose-silica aerogels. Carbohydr. Polym. 2015, 122, 293–300. [Google Scholar] [CrossRef]
- Yang, L.; Zhan, Y.; Gong, Y.; Ren, E.; Lan, J.; Guo, R.; Yan, B.; Chen, S.; Lin, S. Development of eco-friendly CO2-responsive cellulose nanofibril aerogels as “green” adsorbents for anionic dyes removal. J. Hazard. Mater. 2021, 405, 124194. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2021, 9, 104608-1-13. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, M.; Lin, X.; Ren, X.; Huang, T.S.; Kim, I.S. Functional nanocomposite aerogels based on nanocrystalline cellulose for selective oil/water separation and antibacterial applications. Chem. Eng. J. 2019, 371, 306–313. [Google Scholar] [CrossRef]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirtaghavi, A.; Luo, J.; Muthuraj, R. Recent Advances in Porous 3D Cellulose Aerogels for Tissue Engineering Applications: A Review. J. Compos. Sci. 2020, 4, 152. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Sharratt, W.N.; Lopez, C.G.; Sarkis, M.; Tyagi, G.; O’Connell, R.; Rogers, S.E.; Cabral, J.T. Ionotropic Gelation Fronts in Sodium Carboxymethyl Cellulose for Hydrogel Particle Formation. Gels 2021, 7, 44. [Google Scholar] [CrossRef]
- Mandin, S.; Moreau, S.; Talantikite, M.; Novalès, B.; Maigret, J.-E.; Cathala, B.; Moreau, C. Cellulose Nanofibrils/Xyloglucan Bio-Based Aerogels with Shape Recovery. Gels 2021, 7, 5. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Arantes, A.C.C.; das Graças Almeida, C.; Dauzacker, L.C.L.; Bianchi, M.L.; Wood, D.F.; Williams, T.G.; Orts, W.J.; Tonoli, G.H.D. Renewable hybrid nanocatalyst from magnetite and cellulose for treatment of textile effluents. Carbohydr. Polym. 2017, 163, 101–107. [Google Scholar] [CrossRef]
- Pinto, R.; Neves, M.; Neto, P.C.; Trindade, T. Composites of Cellulose and Metal Nanoparticles. In Nanocomposites–New Trends and Developments; Ebrahimi, F., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0762-0. Available online: https://www.intechopen.com/books/nanocomposites-new-trends-and-developments/composites-of-cellulose-and-metal-nanoparticles (accessed on 1 July 2021). [CrossRef] [Green Version]
- Cárdenas-Triviño, G.; Saludes-Betanzo, M.J.; Vergara-González, L. Bactericides Properties of Chitosan Metal Quantum Dots Microbial Pathogenicity against E. coli, S. aureus, and S. Typhi. Int. J. Biol. Macromol. 2017, 104, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Rubina, M.; Shulenina, A.; Svetogorov, R.; Vasilkov, A. Metal-Chitosan Nanocomposites: A Perspective Way to Preparation, Morphology, and Structural Studies. Macromol. Symp. 2020, 389, 1–3. [Google Scholar] [CrossRef]
- Rubina, M.S.; Said-Galiev, E.E.; Naumkin, A.V.; Shulenina, A.V.; Belyakova, O.A.; Vasil’kov, A.Y. Preparation and characterization of biomedical collagen–chitosan scaffolds with entrapped ibuprofen and silver nanoparticles. Polym. Eng. Sci. 2019, 59, 1–4. [Google Scholar] [CrossRef]
- Vasil’kov, A.Y.; Rubina, M.S.; Naumkin, A.V.; Zubavichus, Y.V.; Belyakova, O.A.; Maksimov, Y.V.; Imshennik, V.K. Metal-containing systems based on chitosan and a collagen-chitosan composite. Russ. Chem. Bull. 2015, 64, 1663–1670. [Google Scholar] [CrossRef]
- Rubina, M.S.; Pigaleva, M.A.; Butenko, I.E.; Budnikov, A.V.; Naumkin, A.V.; Gromovykh, T.I.; Lutsenko, S.V.; Vasil’kov, A.Y. Effect of Interaction of Bacterial Cellulose with Gold Nanoparticles Obtained by Metal Vapor Synthesis. Dokl. Phys. Chem. 2019, 488, 146–150. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Dovnar, R.; Smotryn, S.; Iaskevich, N.; Naumkin, A. Plasmon Resonance of Silver Nanoparticles as a Method of Increasing Their Antibacterial Action. Antibiotics 2018, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Rubina, M.S.; Elmanovich, I.V.; Shulenina, A.V.; Peters, G.S.; Svetogorov, R.D.; Egorov, A.A.; Naumkin, A.V.; Vasil’kov, A.Y. Chitosan aerogel containing silver nanoparticles: From metal-chitosan powder to porous material. Polym. Test. 2020, 86, 106481. [Google Scholar] [CrossRef]
- Abd-elsalam, K.A.; Alghuthaymi, M.A.; Shami, A.; Rubina, M.S.; Abramchuk, S.S.; Shtykova, E.V.; Vasil’kov, A.Y. Copper-Chitosan Nanocomposite Hydrogels Against Aflatoxigenic Aspergillus flavus from Dairy Cattle Feed. J. Fungi 2020, 6, 112. [Google Scholar] [CrossRef]
- Rubina, M.S.; Vasil’kov, A.Y.; Naumkin, A.V.; Shtykova, E.V.; Abramchuk, S.S.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. J. Nanostruct. Chem. 2017, 7, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Thermal conductivity/structure correlations in thermal super-insulating pectin aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iα from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; Zhang, Q.; French, A.D.; Wu, Q. Characterization of cellulose I/II hybrid fibers isolated from energycane bagasse during the delignification process: Morphology, crystallinity and percentage estimation. Carbohydr. Polym. 2015, 133, 438–447. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef]
- Laity, P.; Cassidy, A.; Skepper, J.; Jones, B.; Cameron, R. Investigation into the intragranular structures of microcrystalline cellulose and pre-gelatinised starch. Eur. J. Pharm. Biopharm. 2010, 74, 377–387. [Google Scholar] [CrossRef]
- Edgar, C.D.; Gray, D.G. Smooth model cellulose I surfaces from nanocrystal suspensions. Cellulose 2003, 10, 299–306. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Andrade, F.K.; Alves, C.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Johansson, L.S.; Campbell, J.M. Reproducible XPS on biopolymers: Cellulose studies. Surf. Interface Anal. 2004, 36, 1018–1022. [Google Scholar] [CrossRef]
- Hua, X.; Kaliaguine, S.; Kokta, B.V.; Adnot, A. Surface analysis of explosion pulps by ESCA Part 1. Carbon (1s) spectra and oxygen-to-carbon ratios. Wood Sci. Technol. 1993, 27, 449–459. [Google Scholar] [CrossRef]
- Gilbert Carlsson, C.M.; Ström, G. Reduction and Oxidation of Cellulose Surfaces by Means of Cold Plasma. Langmuir 1991, 7, 2492–2497. [Google Scholar] [CrossRef]
- Adel, A.M.; El-Wahab, Z.H.A.; Ibrahim, A.A.; Al-Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Czeremuszkin, G.; Sapieha, S.; Gandini, A. Surface by XPS characterization and inverse gas of cellulose fibres chromatography. Cellulose 1995, 2, 145–157. [Google Scholar] [CrossRef]
- Rubina, M.S.; Pigaleva, M.A.; Naumkin, A.V.; Gromovykh, T.I. Bacterial Cellulose Film Produced by Gluconacetobacter hansenii as a Source Material for Oxidized Nanofibrillated Cellulose. Dokl. Phys. Chem. 2020, 493, 127–131. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Metal nanoparticles and their assemblies. Chem. Soc. Rev. 2000, 29, 27–35. [Google Scholar] [CrossRef]
- Lopez-Salido, I.; Lim, D.C.; Dietsche, R.; Bertram, N.; Kim, Y.D. Electronic and geometric properties of Au nanoparticles on Highly Ordered Pyrolytic Graphite (HOPG) studied using X-ray Photoelectron Spectroscopy (XPS) and Scanning Tunneling Microscopy (STM). J. Phys. Chem. B 2006, 110, 1128–1136. [Google Scholar] [CrossRef]
- Li, R.; He, M.; Li, T.; Zhang, L. Preparation and properties of cellulose/silver nanocomposite fibers. Carbohydr. Polym. 2015, 115, 269–275. [Google Scholar] [CrossRef]
- Li, S.M.; Jia, N.; Ma, M.G.; Zhang, Z.; Liu, Q.H.; Sun, R.C. Cellulose-silver nanocomposites: Microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr. Polym. 2011, 86, 441–447. [Google Scholar] [CrossRef]
- Kheiker, D.M.; Kovalchuk, M.V.; Korchuganov, V.N.; Shilin, Y.N.; Shishkov, V.A.; Sulyanov, S.N.; Dorovatovskiǐ, P.V.; Rubinsky, S.V.; Rusakov, A.A. Station for X-ray structural analysis of materials and single crystals (including nanocrystals) on a synchrotron radiation beam from the wiggler at the Siberia-2 storage ring. Crystallogr. Rep. 2007, 52, 1108–1115. [Google Scholar] [CrossRef]
- Korneev, V.N.; Shlektarev, V.A.; Zabelin, A.V.; Aul’chenko, V.M.; Tolochko, B.P.; Sharafutdinov, M.R.; Medvedev, B.I.; Gorin, V.P.; Nayda, O.V.; Vazina, A.A. New version of the small-angle X-ray equipment for studying biological structures at the station DICSI in the Kurchatov center of synchrotron radiation and nanotechnologies. J. Surf. Investig. 2008, 2, 872–878. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Häusermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Ingham, J.B.; Li, H.; Allen, E.L.; Toney, M.F. SAXSFit: A program for fitting small-angle x-ray and neutron scattering data. arXiv 2009, arXiv:0901.4782. [Google Scholar]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural Materials Science end-station at the Kurchatov Synchrotron Radiation Source: Recent instrumentation upgrades and experimental results. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Naumkin, C.J.P.A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 1 July 2021).
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers. The Scientia ESCA300 Database; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]














| Sample | Volume Shrinkage, % | Bulk Density, cm3 g−1 | Total Pore Volume, cm3 g−1 | Porosity, % |
|---|---|---|---|---|
| AC (aerogel from pristine MCC) | 19 | 0.15 | 6.00 | 90 |
| Ag-AC (aerogel from MCC modified with Ag NPs) | 19 | 0.16 | 5.58 | 89 |
| Sample | SSA, m2g−1 | BJH Cumulative Pore Volume, cm−3g−1 | BJH Mean Pore Dimeter, nm |
|---|---|---|---|
| AC (aerogel from pristine MCC) | 205 | 1.3 | 23 |
| Ag-AC (aerogel from MCC modified with Ag NPs) | 212 | 1.2 | 26 |
| Sample | N | R, Å | σ2, Å2 | ΔE, eV | Rf |
|---|---|---|---|---|---|
| Ag foil | 12 | 2.881 | 0.0096 | 3.0 | 0.010 |
| Ag-MCC | 9.9 | 2.867 | 0.0096 | 2.4 | 0.009 |
| Ag-AC | 9.8 | 2.844 | 0.0096 | −1.7 | 0.005 |
| Sample | Power-Law Slope, α | R, nm | Dispersion, % |
|---|---|---|---|
| MCC | 3.35 | - | - |
| AC | 2.10 | 2.5 | 50 |
| Ag-MCC | 3.45 | 11.0 | 50 |
| Ag-AC; | 3.10 | 10.8 | 50 |
| Sample | Concentration, at.% | |||
|---|---|---|---|---|
| C | O | Ag | O/C | |
| MCC | 63.5 | 36.5 | - | 0.6 |
| Ag-MCC | 63.1 | 36.4 | 0.5 | 0.6 |
| AC | 54.8 | 45.2 | - | 0.8 |
| Ag-AC | 58.7 | 41.2 | 0.01 | 0.7 |
| Sample | Carbon Type | Binding Energies, eV | Peak Width, eV | Relative Intensity |
|---|---|---|---|---|
| MCC | C1 | 285.14 | 1.16 | 0.29 |
| C2 | 286.73 | 1.24 | 0.50 | |
| C3 | 288.02 | 1.24 | 0.10 | |
| C4 | 288.96 | 1.69 | 0.12 | |
| Ag-MCC | C1 | 285.11 | 1.13 | 0.28 |
| C2 | 286.73 | 1.20 | 0.52 | |
| C3 | 288.03 | 1.20 | 0.10 | |
| C4 | 288.95 | 1.20 | 0.10 | |
| AC | C1 | 285.01 | 1.60 | 0.06 |
| C2 | 286.73 | 1.19 | 0.71 | |
| C3 | 288.02 | 1.19 | 0.14 | |
| C4 | 288.65 | 1.59 | 0.09 | |
| Ag-AC | C1 | 284.94 | 1.15 | 0.19 |
| C2 | 286.73 | 1.27 | 0.61 | |
| C3 | 288.02 | 1.27 | 0.12 | |
| C4 | 288.63 | 1.69 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasil’kov, A.; Rubina, M.; Naumkin, A.; Buzin, M.; Dorovatovskii, P.; Peters, G.; Zubavichus, Y. Cellulose-Based Hydrogels and Aerogels Embedded with Silver Nanoparticles: Preparation and Characterization. Gels 2021, 7, 82. https://doi.org/10.3390/gels7030082
Vasil’kov A, Rubina M, Naumkin A, Buzin M, Dorovatovskii P, Peters G, Zubavichus Y. Cellulose-Based Hydrogels and Aerogels Embedded with Silver Nanoparticles: Preparation and Characterization. Gels. 2021; 7(3):82. https://doi.org/10.3390/gels7030082
Chicago/Turabian StyleVasil’kov, Alexander, Margarita Rubina, Alexander Naumkin, Mikhail Buzin, Pavel Dorovatovskii, Georgy Peters, and Yan Zubavichus. 2021. "Cellulose-Based Hydrogels and Aerogels Embedded with Silver Nanoparticles: Preparation and Characterization" Gels 7, no. 3: 82. https://doi.org/10.3390/gels7030082
APA StyleVasil’kov, A., Rubina, M., Naumkin, A., Buzin, M., Dorovatovskii, P., Peters, G., & Zubavichus, Y. (2021). Cellulose-Based Hydrogels and Aerogels Embedded with Silver Nanoparticles: Preparation and Characterization. Gels, 7(3), 82. https://doi.org/10.3390/gels7030082