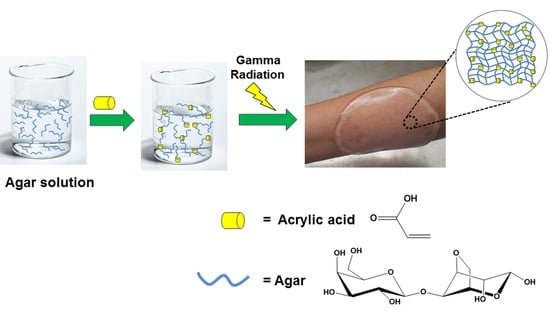
Highly Crosslinked Agar/Acrylic Acid Hydrogels with Antimicrobial Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. FTIR-ATR Analysis
2.1.2. 13C-NMR Analysis
2.1.3. Scanning Electron Microscopy (SEM)
2.1.4. Thermal Analysis
2.1.5. Mechanical Properties
2.2. Swelling Studies
2.3. Critical pH
2.4. Antimicrobial Activity
3. Conclusions
4. Materials and Methods
4.1. Materials and Methods
4.2. Preparation of Hydrogels
4.3. Synthesis of AgNPs
4.4. Structural Characterization
4.5. Thermal Analysis
4.6. Swelling Studies and Critical pH
4.7. Load of AgNPs
4.8. Antimicrobial Assay Using E. coli and MRSA
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J.S. Smart polymers and their applications as biomaterials. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R.L., Chiellini, E., Eds.; University of Oulu: Oulu, Finland, 2007; Volume 3, pp. 2–27. [Google Scholar]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel dressings for the treatment of burn wounds: An Up-To-Date Overview. Materials 2020, 13, 2853–2876. [Google Scholar]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burn. Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Y.; Luo, X.; Lu, A.; Li, Y.; Li, B.; Liu, S. O/W pickering emulsion templated organo-hydrogels with enhanced mechanical strength and energy storage capacity. ACS Appl. Bio Mater. 2019, 2, 480–487. [Google Scholar]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar]
- Elyashevich, G.K.; Bel’nikevich, N.G.; Vesnebolotskaya, S.A. Swelling–contraction of sodium polyacrylate hydrogels in media with various pH values. Polym. Sci. 2009, 51, 809–812. [Google Scholar]
- Zhang, R.; Ruan, H.; Zhou, T.; Fu, Q.; Peng, H.; Zhu, X.; Yao, Y. High-performance poly(acrylic acid) hydrogels formed with block copolymer crosslinker containing amino-acid derivatives. Soft Matter 2019, 15, 7381–7389. [Google Scholar] [CrossRef]
- Rapado Raneque, M.; Rodriguez Rodriguez, A.; Peniche Covas, C. Hydrogel wound dressing preparation at laboratory scale by using electron beam and gamma radiation. Nucleus 2013, 53, 24–31. [Google Scholar]
- Wang, M.; Xu, L.; Hu, H.; Zhai, M.; Peng, J.; Nho, Y.; Li, J.; Wei, G. Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl. Instrum. Meth. B 2007, 265, 385–389. [Google Scholar]
- Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. II. Effect of some factors on radiation-induced crosslinking of CMC. J. Appl. Polym. Sci. 2001, 81, 3030–3037. [Google Scholar] [CrossRef]
- Bucio, E.; Burillo, G. Radiation-induced grafting of sensitive polymers. J. Radioanal. Nucl. Chem. 2009, 280, 239–243. [Google Scholar] [CrossRef]
- Liu, P.; Zhai, M.; Li, J.; Peng, J.; Wu, J. Radiation preparation and swelling behavior of sodium carboxymethyl cellulose hydrogels. Radiat. Phys. Chem. 2002, 63, 525–528. [Google Scholar] [CrossRef]
- Zhang, R.; Ruan, H.; Fu, Q.; Zhu, X.; Yao, Y. A high strain, adhesive, self-healable poly(acrylic acid) hydrogel with temperature sensitivity as an epidermal sensor. Mater. Adv. 2020, 1, 329–333. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Audifred-Aguilar, J.C.; Sanchez-Obregon, R.; Bucio, E. Antimicrobial polyurethane catheters synthesized by grafting-radiation method doped with silver nanoparticles. React. Funct. Polym. 2021, 167, 105006. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Mechanisms of mucoadhesion of poly (acrylic acid) hydrogels. Pharm. Res. 1987, 4, 457–464. [Google Scholar] [CrossRef]
- Lu, F.; Wang, Y.; Wang, C.; Kuga, S.; Huang, Y.; Wu, M. Two-dimensional nanocellulose-enhanced high-strength, self-adhesive, and strain-sensitive poly(acrylic acid) hydrogels fabricated by a radical-induced strategy for a skin sensor. ACS Sustain. Chem. Eng. 2020, 8, 3427–3436. [Google Scholar] [CrossRef]
- Baranovskiia, V.Y.; Ganeva, V.G.; Petkovab, V.B.; Voichevaa, K.C.; Dimitrov, M.V. Hydrogels based on polycarboxylic acid–agar agar complexes. Colloid J. 2012, 74, 645–648. [Google Scholar] [CrossRef]
- Awadhiya, A.; Kumar, D.; Verma, V. Crosslinking of agarose bioplastic using citric acid. Carbohydr. Polym. 2016, 151, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Yin, Z.C.; Wang, Y.L.; Wang, K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J. Drug Deliv. Sci. Tech. 2018, 43, 12–18. [Google Scholar]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.C.; King, D.M.; Thomas, C.B. The oxidation of some polysaccharides by the hydroxyl radical: An e.s.r. investigation. Carbohydr. Res. 1984, 125, 217–235. [Google Scholar] [CrossRef]
- Gryczka, U.; Dondi, D.; Chmielewski, A.G.; Migdal, W.; Buttafava, A.; Faucitano, A. The mechanism of chitosan degradation by gamma and e-beam irradiation. Radiat. Phys. Chem. 2009, 78, 543–548. [Google Scholar] [CrossRef]
- Ren, L.; Wang, X.; Li, S.; Li, J.; Zhu, X.; Zhang, L.; Gao, F.; Zhou, G. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int. J. Biol. Macromol. 2018, 120, 641–649. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L.; Borsa, J.; Papp, J.; Hargittai, P.; Korecz, L. Modification of cotton-cellulose by preirradiation grafting. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 259–265. [Google Scholar] [CrossRef]
- Zada, M.H.; Kumar, A.; Elmalak, O.; Mechrez, G.; Domb, A.J. Effect of Ethylene Oxide and Gamma (ƒÁ.) Sterilization on the properties of a PLCL polymer material in balloon implants. ACS Omega 2019, 4, 21319–21326. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kim, E.A.; Gunasekara, U.K.; Abeytunga, D.T.U.; Nanayakkara, C.; De Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, C.C.; Varca, G.H.C.; Ruiz, J.C.; Lopes, P.S.; Mathor, M.B.; Lugão, A.B.; Bucio, E. Radiation-grafting of thermo- and pH-responsive poly(N-vinylcaprolactam-co-acrylic acid) onto silicone rubber and polypropylene films for biomedical purposes. Radiat. Phys. Chem. 2014, 97, 298–303. [Google Scholar] [CrossRef]
- Tomar, R.S.; Gupta, I.; Singhal, R.; Nagpal, A.K. Synthesis of poly (Acrylamide-co-acrylic acid) based superaborbent hydrogels: Study of network parameters and swelling behaviour. Polym. Plast. Technol. Eng. 2007, 46, 481–488. [Google Scholar]
- McNeill, C.; Sadeghi, S.M.T. Thermal stability and degradation mechanisms of poly(acrylic acid) and its salts: Part 1 poly (acrylic acid). Polym. Degrad. Stab. 1990, 29, 233–246. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Flores-Rojas, G.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Graft copolymerization by ionization radiation, characterization, and enzymatic activity of temperature-responsive SR-g-PNVCL loaded with lysozyme. React. Funct. Polym. 2018, 126, 74–82. [Google Scholar]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. Prog. Mol. Environ. Bioeng. 2011, 51, 117–150. [Google Scholar]
- Li, X.; Yang, Q.; Zhao, Y.; Longa, S.; Zheng, J. Dual physically crosslinked double network hydrogels with high toughness and self-healing properties. Soft Matter 2017, 13, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Dubruel, P.; van Vlierberghe, S. Smart polymer hydrogels: Properties, synthesis and applications. In Smart Polymers and Their Applications; Aguilar, M.R., Román, J.S., Eds.; Elsevier: Cambridge, UK, 2014; pp. 237–270. [Google Scholar]
- Pino-Ramos, V.H.; Cedillo, G.; López-Barriguete, E.; Bucio, E. Comonomer effect: Switching the lower critical solution temperature to upper critical solution temperature in thermo-pH sensitive binary graft copolymers. J. Appl. Polym. Sci. 2019, 136, 48170–48178. [Google Scholar] [CrossRef]
- Jubeh, B.; Breijyeh, Z.; Karaman, R. Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef]
- Krajewski, S.; Prucek, R.; Panacek, A.; Avci-Adali, M.; Nolte, A.; Straub, A.; Zboril, R.; Wendel, H.P.; Kvitek, L. Hemocompatibility evaluation of different silver nanoparticle concentrations employing a modified Chandler-loop in vitro assay on human blood. Acta Biomater. 2013, 9, 7460–7468. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Shamova, O.V.; Orlo, D.S.; Pazina, T.Y.; Boldina, A.S.; Kokryakov, V.N. Study of antimicrobial and hemolytic activities of silver nanoparticles prepared by chemical reduction. Glass Phys. Chem. 2010, 36, 628–634. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]











| Sample | Vol. AAc (mL) | Radiation Dose (kGy) | Strain (%) | Strain (mm/mm) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| Hydro1 | 1.0 | 15 | 860.6 | 215.0 | 0.90 |
| Hydro2 | 1.0 | 20 | 524.2 | 131.0 | 0.63 |
| Hydro3 | 1.0 | 25 | 423.8 | 66.6 | 0.81 |
| Hydro4 | 1.5 | 15 | 589.9 | 150.2 | 1.0 |
| Hydro5 | 1.5 | 20 | 155.0 | 46.0 | 1.2 |
| Hydro6 | 1.5 | 25 | 360.0 | 44.6 | 1.9 |
| Hydro7 | 2.0 | 15 | 383.4 | 90.9 | 3.04 |
| Hydro8 | 2.0 | 20 | 268.0 | 56.1 | 3.0 |
| Hydro9 | 2.0 | 25 | 180.6 | 27.6 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino-Ramos, V.H.; Duarte-Peña, L.; Bucio, E. Highly Crosslinked Agar/Acrylic Acid Hydrogels with Antimicrobial Properties. Gels 2021, 7, 183. https://doi.org/10.3390/gels7040183
Pino-Ramos VH, Duarte-Peña L, Bucio E. Highly Crosslinked Agar/Acrylic Acid Hydrogels with Antimicrobial Properties. Gels. 2021; 7(4):183. https://doi.org/10.3390/gels7040183
Chicago/Turabian StylePino-Ramos, Victor H., Lorena Duarte-Peña, and Emilio Bucio. 2021. "Highly Crosslinked Agar/Acrylic Acid Hydrogels with Antimicrobial Properties" Gels 7, no. 4: 183. https://doi.org/10.3390/gels7040183
APA StylePino-Ramos, V. H., Duarte-Peña, L., & Bucio, E. (2021). Highly Crosslinked Agar/Acrylic Acid Hydrogels with Antimicrobial Properties. Gels, 7(4), 183. https://doi.org/10.3390/gels7040183