Bioinspired Remineralization of Artificial Caries Lesions Using PDMAEMA/Carbomer/Calcium Phosphates Hybrid Microgels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Hybrid PDMAEMA/Carbomer 940/CaP Microgels
2.1.1. TEM Characterization of Hybrid PDMAEMA/Carbomer 940/CaP Microgels
2.1.2. NMR
NMR Characterization of Neat CaP Phase (Obtained without Polymer)
Single Pulse 31P NMR Spectrum of the Hybrid PDMAEMA/Carbomer 940/CaP Microgels
NMR Characterization of CaP Phase Obtained via In Situ Precipitation in the Hybrid PDMAEMA/Carbomer 940/CaP Microgels
2.1.3. IR Spectra Characterization of PDMAEMA/Carbomer 940/CaP Hybrid Microgels
2.2. Characterization of Demineralization and Remineralization of Enamel
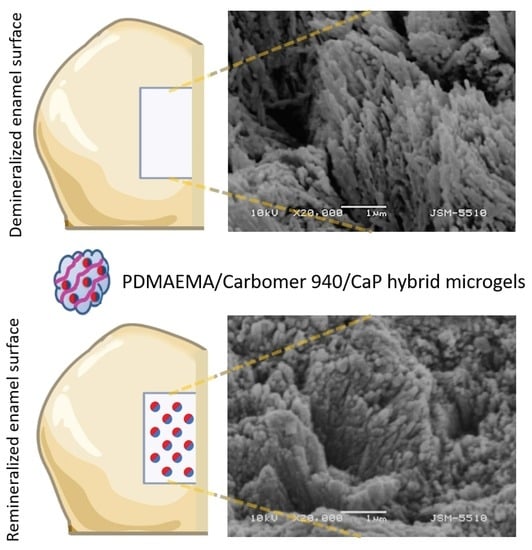
2.2.1. SEM Characterization of Treated Tooth Surfaces
SEM Characterization of Artificial Caries Lesions
SEM Characterization of Artificial Caries Lesions Remineralized with Hybrid PDMAEMA/Carbomer 940/CaP Microgels
- directly from ACP (ACP→HAP) through direct structure reconstruction with partial dissolution that takes place during maturation;
- the transformation of OCP to the HAP phase can proceed either by a dissolution–precipitation reaction, or by a direct solid transformation. The structural similarity of the OCP and HAP structures enables HAP to grow epitaxially on the (100) surface of OCP [34];
- via dissolution and precipitation from DCPD (DCPD→HAP); as well as
- via β-TCP due to its excellent bioresorption.
2.2.2. X-ray Diffraction of De- and Remineralized Enamel Lesions
2.2.3. Infrared Spectroscopy Characterization of Remineralized Artificial Caries Lesions
2.2.4. Raman Spectra of the Remineralized Artificial Caries Lesions
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Remineralization System
4.2.1. Synthesis of Linear PDMAEMA
4.2.2. Synthesis of PDMAEMA/Carbomer 940/Calcium Phosphate (CaP) Hybrid Microgels
4.3. Preparation of Demineralized Enamel Lesions
4.4. Remineralization of the Artificial Enamel Lesions with PDMAEMA/Carbomer 940/CaP Hybrid Microgels
4.5. Scanning Electron Microscopy (SEM)
4.6. Transmission Electron Microscopy
4.7. Infrared Spectroscopy (FT-IR)
4.8. Nuclear Magnetic Resonance (NMR)
4.9. X-ray Diffraction (XRD)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 8, 4754–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [Green Version]
- Marie, B.; Joubert, C.; Tayalé, A.; Zanella-Cléon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different secretory repertoires control he biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Shellis, R.P. Relationship between human enamel structure and the formation of caries-like lesions in vitro. Arch. Oral Biol. 1984, 29, 975–981. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Chaffee, B.W. The evidence for caries management by risk assessment (CAMBRA®). Adv Dent Res. 2018, 29, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.C.; McLean, M.E. Minimally invasive operative care. Part 1: Minimal intervention and concepts for minimally invasive cavities. J. Adhes. Dent. 2001, 3, 7–16. Available online: http://www.quintpub.com/userhome/jad/jad_3_1_peters_2.pdf (accessed on 5 May 2022).
- Alkilzy, M.; Tarabaih, A.; Splieth, C.H. Efficacy, clinical applicability and safety of Curodont TM Repair in children with early occlusal caries. Caries Res. 2015, 49, 311. [Google Scholar]
- Divaris, K.; Preisser, J.S.; Slade, G.D. Surface-specific efficacy of fluoride varnish in caries prevention in the primary dentition: Results of a community randomized clinical trial. Caries Res. 2013, 47, 78–87. [Google Scholar] [CrossRef]
- Anderson, M.; Dallhof, G.; Twetman, S.; Jansson, L.; Bergenlid, A.C.; Grindefjord, M. Effectiveness of early preventive intervention with semiannual fluoride varnish application in toddlers living in high-risk areas: A stratified cluster-randomized controlled trial. Caries Res. 2016, 50, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Weatherell, J.A.; Deutsch, D.; Robinson, C.; Hallsworth, A.S. Assimilation of fluoride by enamel throughout the life of the tooth. Caries Res. 1977, 11 (Suppl. S1), 85–115. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T. Remineralization Therapies for Initial Caries Lesions. Curr. Oral Health Rep. 2015, 2, 95–101. [Google Scholar] [CrossRef]
- Ruan, Q.; Moradian-Oldak, J. Amelogenin and enamel biomimetics. J. Mater. Chem. B. 2015, 3, 3112–3129. [Google Scholar] [CrossRef]
- Fang, P.A.; Conway, J.F.; Margolis, H.C.; Simmer, J.P.; Beniash, E. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc. Natl. Acad. Sci. USA 2011, 108, 14097–14102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cölfen, H. Bio-inspired Mineralization Using Hydrophilic Polymers. In Biomineralization II. Topics in Current Chemistry; Naka, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 271. [Google Scholar] [CrossRef]
- Rawlinson, L.-A.B.; Ryan, S.M.; Mantovani, G.; Syrett, J.A.; Haddleton, D.M.; Brayden, D.J. Antibacterial Effects of Poly(2-(dimethylamino ethyl)methacrylate) against Selected Gram-Positive and Gram-Negative Bacteria. Biomacromolecules 2010, 11, 443–453. [Google Scholar] [CrossRef]
- Magennis, E.P.; Francini, N.; Mastrotto, F.; Catania, R.; Redhead, M.; Fernandez-Trillo, F.; Mantovani, G. Polymers for binding of the gram-positive oral pathogen Streptococcus mutans. PLoS ONE 2017, 12, e0180087. [Google Scholar] [CrossRef] [Green Version]
- Keely, S.; Ryan, S.M.; Haddleton, D.M.; Limer, A.; Mantovani, G.; Murphy, E.P.; Colgan, S.P.; Brayden, D.J. Dexamethasone-pDMAEMA polymeric conjugates reduce inflammatory biomarkers in human intestinal epithelial monolayers. J. Control Release 2009, 135, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Volkmer, T.; Magalhães, J.; Sousa, V.; Santos, L.; Burguera, E.; Blanco, F.; Rodríguez-Lorenzo, L. 2-(Dimethylamino)ethyl Methacrylate/(2-Hydroxyethyl) Methacrylate/α-Tricalcium Phosphate Cryogels for Bone Repair, Preparation and Evaluation of the Biological Response of Human Trabecular Bone-Derived Cells and Mesenchymal Stem Cells. Polymers 2014, 6, 2510–2525. [Google Scholar] [CrossRef] [Green Version]
- Lefrancois, P.; Ibarboure, E.; Payre, B.; Gontier, E.; Le Meins, J.-F.; Schatz, C. Insights into Carbopol gel formulations: Microscopy analysis of the microstructure and the influence of polyol additives. J. Appl. Polym. Sci. 2015, 132, 42761. [Google Scholar] [CrossRef]
- Gebauer, D. How Can Additives Control the Early Stages of Mineralisation? Minerals 2018, 8, 179. [Google Scholar] [CrossRef]
- Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. PNAS 2004, 101, 12096–12101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowker, S.E.P.; Elliott, J.C.; Davis, G.R.; Wassif, H.S. Longitudinal Study of the Three-Dimensional Development of Subsurface Enamel Lesions during in vitro Demineralization. Caries Res. 2002, 37, 237–245. [Google Scholar] [CrossRef]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. A Review of the Common Models Used in Demineralization-Remineralization for Cariology Research. Dent. J. 2017, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 2009, 30, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Risnes, S.; Li, C. On the method of revealing enamel structure by acid etching. Aspects of optimization and interpretation. Microsc. Res. Tech. 2019, 82, 1668–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Featherstone, J.D.; Goodmen, P.; Mclean, J.D. Electron microscopy study of defect zones in dental enamel. J. Ultrastruct. Res. 1979, 67, 117–123. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Miake, Y. High-resolution electron microscopy of enamel-crystal demineralization and remineralization in carious lesions. J. Elec. Micr. 2003, 52, 605–613. [Google Scholar] [CrossRef]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef] [Green Version]
- DeVol, R.T.; Sun, C.-Y.; Marcus, M.A.; Coppersmith, S.N.; Myneni, S.C.B.; Gilbert, P.U.P.A. Nanoscale transforming mineral phases in fresh nacre. J. Am. Chem. Soc. 2015, 137, 13325–13333. [Google Scholar] [CrossRef]
- Mass, T.; Giuffre, A.J.; Sun, C.-Y.; Stifler, C.A.; Frazier, M.J.; Neder, M.; Tamura, N.; Stan, C.V.; Marcus, M.A.; Gilbert, P.U.P.A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2018, 114, E7670–E7678. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, L.; Wang, H.; Zhang, W.; Li, J.; Liu, D.; Han, F.; Chen, S.; Li, B. Calcium phosphate bone cement with enhanced physicochemical properties via in situ formation of an interpenetrating network. J. Mater. Chem. B 2015, 3, 5318–5329. [Google Scholar] [CrossRef]
- Moshy, S.E.; Abbass, M.M.S.; El-Motayam, A.M. Biomimetic remineralization of acid etched enamel using agarose hydrogel model. F1000 Res. 2018, 7, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, S.; Anderson, P.; Al-Jawad, M. Recovery of Crystallographic Texture in Remineralized Dental Enamel. PLoS ONE 2014, 9, e108879. [Google Scholar] [CrossRef]
- Yin, Y.; Yun, S.; Fang, J.; Chen, H. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem. Commun. 2009, 39, 5892–5894. [Google Scholar] [CrossRef]
- Titorenkova, R.; Dyulgerova, E.; Petkova, V.; Ilieva, R. Carbonation and dehydroxylation of apatite during high energy milling of biphasic Ca-phosphate ceramics. Cer. Int. 2019, 45, 7025–7033. [Google Scholar] [CrossRef]
- Jegova, G.; Titorenkova, R.; Rashkova, M.; Mihailova, B. Raman and IR reflection micro-spectroscopic study of Er:YAG laser treated permanent and deciduous human teeth. J. Raman Spectrosc. 2013, 44, 1483–1490. [Google Scholar] [CrossRef]
- Gadaleta, S.J.; Paschalis, E.P.; Betts, F.; Mendelsohn, R.; Boskey, A.L. Fourier Transform Infrared Spectroscopy of the Solution-Mediated Conversion of Amorphous Calcium Phosphate to Hydroxyapatite: New Correlations Between X-Ray Diffraction and Infrared Data. Calcif. Tissue Int. 1996, 58, 9–16. [Google Scholar] [CrossRef]
- Rabadjieva, D.; Sezanova, K.; Gergulova, R.; Titorenkova, R.; Tepavitcharova, S. Precipitation and phase transformation of dicalcium phosphate dihydrate in electrolyte solutions of simulated body fluids: Thermodynamic modelling and kinetic studies. J. Biom. Mat. Res. 2020, 108, 1607–1616. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Celve, S.; Alonson, B.; Durand, J.O.; Bujoli, B.; Gan, Z.H.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]












| Enamel Sample | Native | Demineralized | Remineralized |
|---|---|---|---|
| Crystallite size (Å) | 153 | 184 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonchev, A.; Simeonov, M.; Shestakova, P.; Vasileva, R.; Titorenkova, R.; Apostolov, A.; Dyulgerova, E.; Vassileva, E. Bioinspired Remineralization of Artificial Caries Lesions Using PDMAEMA/Carbomer/Calcium Phosphates Hybrid Microgels. Gels 2022, 8, 681. https://doi.org/10.3390/gels8100681
Bonchev A, Simeonov M, Shestakova P, Vasileva R, Titorenkova R, Apostolov A, Dyulgerova E, Vassileva E. Bioinspired Remineralization of Artificial Caries Lesions Using PDMAEMA/Carbomer/Calcium Phosphates Hybrid Microgels. Gels. 2022; 8(10):681. https://doi.org/10.3390/gels8100681
Chicago/Turabian StyleBonchev, Alexander, Marin Simeonov, Pavletta Shestakova, Radosveta Vasileva, Rositsa Titorenkova, Anton Apostolov, Elena Dyulgerova, and Elena Vassileva. 2022. "Bioinspired Remineralization of Artificial Caries Lesions Using PDMAEMA/Carbomer/Calcium Phosphates Hybrid Microgels" Gels 8, no. 10: 681. https://doi.org/10.3390/gels8100681
APA StyleBonchev, A., Simeonov, M., Shestakova, P., Vasileva, R., Titorenkova, R., Apostolov, A., Dyulgerova, E., & Vassileva, E. (2022). Bioinspired Remineralization of Artificial Caries Lesions Using PDMAEMA/Carbomer/Calcium Phosphates Hybrid Microgels. Gels, 8(10), 681. https://doi.org/10.3390/gels8100681