Effect of Composite Chitosan/Sodium Alginate Gel Coatings on the Quality of Fresh-Cut Purple-Flesh Sweet Potato
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Coatings on the Color Change during Storage
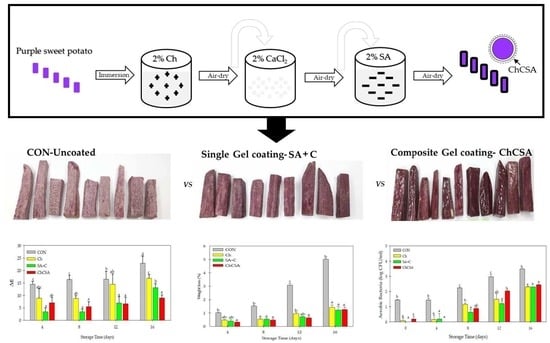
2.2. Effect of Coatings on Weight Loss during Storage
2.3. Effect of Composite Edible Coatings on Firmness
2.4. Effect of Composite Edible Coatings on Microbial Growth
2.5. Effect of Coatings on CO2 Production
2.6. Effect of Coatings on Soluble Solid Concentration and pH
2.7. Effect of Coatings on Total Anthocyanin Content and Total Phenolic Content
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Coating Solutions Preparation
4.3. Sample Preparation
4.4. Coating Application on the Samples
4.5. Color Measurement
4.6. Weight Loss Measurement
4.7. Firmness Measurement
4.8. Microbial Analysis
4.9. Carbon Dioxide Production
4.10. Soluble Solid Concentration and pH
4.11. Sample Extraction for Total Anthocyanin Content and Total Phenolic Content
4.11.1. Total Anthocyanin Content
4.11.2. Total Phenolic Content
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Deng, L.; Chen, J.; Zhou, S.; Liu, S.; Fu, Y.; Yang, C.; Liao, Z.; Chen, M. An analytical pipeline to compare and characterise the anthocyanin antioxidant activities of purple sweet potato cultivars. Food Chem. 2016, 194, 46. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Andersen, Ø. Spectroscopic techniques applied to flavonoids. Flavonoids Chem. Biochem. Appl. 2006, 37–142. [Google Scholar]
- Olawuyi, I.F.; Park, J.J.; Lee, J.J.; Lee, W.Y. Combined effect of chitosan coating and modified atmosphere packaging on fresh-cut cucumber. Food Sci. Nutr. 2019, 7, 1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Han, M.; Xie, Y.; Wang, M.; Cao, C. Application of ethylene-regulating packaging in post-harvest fruits and vegetables storage: A review. Packag. Technol. Sci. 2022, 35, 461. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Poverenov, E. Improving food products’ quality and storability by using Layer by Layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W. Influence of chitosan coating and packaging materials on the quality characteristics of fresh-cut cucumber. Korean J. Food Preserv. 2019, 26, 371. [Google Scholar] [CrossRef] [Green Version]
- Aayush, K.; McClements, D.J.; Sharma, S.; Sharma, R.; Singh, G.P.; Sharma, K.; Oberoi, K. Innovations in the development and application of edible coatings for fresh and minimally processed Apple. Food Control 2022, 141, 109188. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Lee, W.Y. Application of plant mucilage polysaccharides and their techno-functional properties’ modification for fresh produce preservation. Carbohydr. Polym. 2021, 272, 118371. [Google Scholar] [CrossRef]
- Park, D.H.; Park, J.J.; Olawuyi, I.F.; Lee, W.Y. Quality of White mushroom (Agaricus bisporus) under argon-and nitrogen-based controlled atmosphere storage. Sci. Hortic. 2020, 265, 109229. [Google Scholar] [CrossRef]
- Dhall, R. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol. 2014, 7, 1424. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237. [Google Scholar]
- Medeiros, B.G.d.S.; Pinheiro, A.C.; Carneiro-da-Cunha, M.G.; Vicente, A.A. Development and characterization of a nanomultilayer coating of pectin and chitosan–Evaluation of its gas barrier properties and application on ‘Tommy Atkins’ mangoes. J. Food Eng. 2012, 110, 457. [Google Scholar] [CrossRef] [Green Version]
- Brasil, I.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.; Moreira, R. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT-Food Sci. Technol. 2012, 47, 39. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; Gonzalez-Martinez, C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496. [Google Scholar] [CrossRef]
- Acevedo, C.A.; López, D.A.; Tapia, M.J.; Enrione, J.; Skurtys, O.; Pedreschi, F.; Brown, D.I.; Creixell, W.; Osorio, F. Using RGB image processing for designing an alginate edible film. Food Bioprocess Technol. 2012, 5, 1511. [Google Scholar] [CrossRef]
- Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D.; Serrano, M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J. Sci. Food Agric. 2008, 88, 1287. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951. [Google Scholar] [CrossRef]
- Nehchiri, N.; Amiri, S.; Radi, M. Improving the water barrier properties of alginate packaging films by submicron coating with drying linseed oil. Packag. Technol. Sci. 2021, 34, 283. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.; Piette, G.; Begin, A.; Holley, R. Diffusion of acetic and propionic acids from chitosan-based antimicrobial packaging films. J. Food Sci. 2000, 65, 768. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703. [Google Scholar] [CrossRef]
- Han, C.; Lederer, C.; McDaniel, M.; Zhao, Y. Sensory evaluation of fresh strawberries (Fragaria ananassa) coated with chitosan-based edible coatings. J. Food Sci. 2005, 70, S172. [Google Scholar] [CrossRef]
- Chien, P.-J.; Sheu, F.; Yang, F.-H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Quality evaluation of blueberries coated with chitosan and sodium alginate during postharvest storage. Int. Food Res. J. 2017, 24, 1553–1561. [Google Scholar]
- Chiabrando, V.; Giacalone, G. Effect of different coatings in preventing deterioration and preserving the quality of fresh-cut nectarines (cv Big Top). CyTA-J. Food 2013, 11, 285. [Google Scholar] [CrossRef]
- Soison, B.; Jangchud, K.; Jangchud, A.; Harnsilawat, T.; Piyachomkwan, K. Characterization of starch in relation to flesh colors of sweet potato varieties. Int. Food Res. J. 2015, 22, 2302. [Google Scholar]
- Kou, X.; He, Y.; Li, Y.; Chen, X.; Feng, Y.; Xue, Z. Effect of abscisic acid (ABA) and chitosan/nano-silica/sodium alginate composite film on the color development and quality of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao). Food Chem. 2019, 270, 385. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry. LWT Food Sci. Technol. 2010, 43, 837. [Google Scholar] [CrossRef]
- Tapia, M.; Rojas-Graü, M.; Carmona, A.; Rodríguez, F.; Soliva-Fortuny, R.; Martin-Belloso, O. Use of alginate-and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocoll. 2008, 22, 1493. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT-Food Sci. Technol. 2008, 41, 139. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT-Food Sci. Technol. 2008, 41, 1862. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Adzahan, N.M. Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol. Technol. 2014, 88, 1. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.; Cerqueira, M.A.; Texeira, J.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Effect of an edible nanomultilayer coating by electrostatic self-assembly on the shelf life of fresh-cut mangoes. Food Bioprocess Technol. 2015, 8, 647. [Google Scholar] [CrossRef] [Green Version]
- Chiabrando, V.; Giacalone, G. Anthocyanins, phenolics and antioxidant capacity after fresh storage of blueberry treated with edible coatings. Int. J. Food Sci. Nutr. 2015, 66, 248. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009; Volume 9. [Google Scholar]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Mishra, B.; Khatkar, B.; Garg, M.; Wilson, L. Permeability of edible coatings. J. Food Sci. Technol. 2010, 47, 109. [Google Scholar] [CrossRef] [Green Version]
- Ghidelli, C.; Mateos, M.; Rojas-Argudo, C.; Pérez-Gago, M.B. Extending the shelf life of fresh-cut eggplant with a soy protein–cysteine based edible coating and modified atmosphere packaging. Postharvest Biol. Technol. 2014, 95, 81. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Jiang, Y.; Yun, J.; Li, W. Effects of chitosan-based coating and modified atmosphere packaging (MAP) on browning and shelf life of fresh-cut lotus root (Nelumbo nucifera Gaerth). Innov. Food Sci. Emerg. Technol. 2010, 11, 684. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125. [Google Scholar] [CrossRef]
- Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Composite coatings of chitosan and alginate emulsions with olive oil to enhance postharvest quality and shelf life of fresh figs (Ficus carica L. cv.‘Pingo De Mel’). Foods 2021, 10, 718. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Al-Ibresam, O.T.; Kumar, N.; Paidari, S.; Trajkovska Petkoska, A.; Agarwal, V. Physicochemical, Morphological and Functional Characterization of Edible Anthocyanin-Enriched Aloe Vera Coatings on Fresh Figs (Ficus carica L.). Gels 2022, 8, 645. [Google Scholar] [CrossRef]
- Sipahi, R.; Castell-Perez, M.; Moreira, R.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT-Food Sci. Technol. 2013, 51, 9. [Google Scholar] [CrossRef]
- Zam, W. Effect of alginate and chitosan edible coating enriched with olive leaves extract on the shelf life of sweet cherries (Prunus avium L.). J. Food Qual. 2019, 2019, 8192964. [Google Scholar] [CrossRef] [Green Version]
- Qamar, J.; Ejaz, S.; Anjum, M.A.; Nawaz, A.; Hussain, S.; Ali, S.; Saleem, S. Effect of Aloe vera gel, chitosan and sodium alginate based edible coatings on postharvest quality of refrigerated strawberry fruits of cv. Chandler. J. Hortic. Sci. Technol. 2018, 1, 8. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Hancock, J.F.; Berkheimer, S.; Hanson, E.J. Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J. Agric. Food Chem. 2002, 50, 893. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chit, C.-S.; Olawuyi, I.F.; Park, J.J.; Lee, W.Y. Effect of Composite Chitosan/Sodium Alginate Gel Coatings on the Quality of Fresh-Cut Purple-Flesh Sweet Potato. Gels 2022, 8, 747. https://doi.org/10.3390/gels8110747
Chit C-S, Olawuyi IF, Park JJ, Lee WY. Effect of Composite Chitosan/Sodium Alginate Gel Coatings on the Quality of Fresh-Cut Purple-Flesh Sweet Potato. Gels. 2022; 8(11):747. https://doi.org/10.3390/gels8110747
Chicago/Turabian StyleChit, Chit-Swe, Ibukunoluwa Fola Olawuyi, Jong Jin Park, and Won Young Lee. 2022. "Effect of Composite Chitosan/Sodium Alginate Gel Coatings on the Quality of Fresh-Cut Purple-Flesh Sweet Potato" Gels 8, no. 11: 747. https://doi.org/10.3390/gels8110747
APA StyleChit, C. -S., Olawuyi, I. F., Park, J. J., & Lee, W. Y. (2022). Effect of Composite Chitosan/Sodium Alginate Gel Coatings on the Quality of Fresh-Cut Purple-Flesh Sweet Potato. Gels, 8(11), 747. https://doi.org/10.3390/gels8110747