Development of Thermoresponsive-Gel-Matrix-Embedded Amoxicillin Trihydrate-Loaded Bovine Serum Albumin Nanoparticles for Local Intranasal Therapy
Abstract
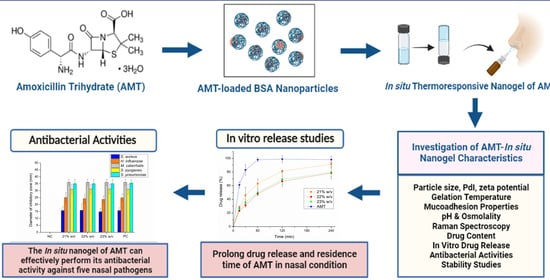
:1. Introduction
2. Results and Discussion
2.1. Optimization of Preparation of AMT-BSA Nanoparticles
2.2. Preparation of In Situ Gelling Thermosensitive Nasal Gel
2.3. Determination of Gelling Temperature and Gelling Time of Formulations
2.4. Mucoadhesive Properties of Nasal Formulations
2.5. Average Hydrodynamic Diameter, PdI, and Zeta Potential
2.6. pH and Osmolality of Optimized Formulations
2.7. Raman Mapping of Drug Distribution
2.8. Drug Content
2.9. In Vitro Drug Release Study
2.10. Antibacterial Activity Studies
2.11. Stability Studies of AMT in the In-Situ Thermosensitive Preparation
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Optimization of Amoxicillin-Loaded Albumin-Based Nanoparticles
4.3. Preparation of Nanogel Formulation
4.4. Dynamic Light Scattering and Zeta Potential Determination
4.5. Rheological Studies
4.6. Mucoadhesive Strength
4.7. Measurement of pH and Osmolality
4.8. Raman Spectroscopy
4.9. Drug Content Measurement
4.10. HPLC Analysis
4.11. In Vitro Drug Release Study
4.12. In Vitro Antibacterial Activities
4.12.1. Bacterial Strains
4.12.2. In Vitro Antibacterial Activity of In Situ Gelling Thermoresponsive Nasal Formulations
4.13. Stability Studies of In-Situ Gelling Thermoresponsive Preparations
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masood, A.; Moumoulidis, I.; Panesar, J. Acute Rhinosinusitis in Adults: An Update on Current Management. Postgrad. Med. J. 2007, 83, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokkens, W.; Lund, V.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Al, E. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012, 23, 1–298. [Google Scholar]
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Ashok Kumar, K.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A.; et al. Clinical Practice Guideline (Update): Adult Sinusitis. Otolaryngol. Head Neck Surg. USA 2015, 152, S1–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, E.R.; Applegate, K.E.; Bordley, C.; Darrow, D.H.; Glode, M.P.; Marcy, S.M.; Nelson, C.E.; Rosenfeld, R.M.; Shaikh, N.; Smith, M.J.; et al. Clinical Practice Guideline for the Diagnosis and Management of Acute Bacterial Sinusitis in Children Aged 1 to 18 Years. Pediatrics 2013, 132, e262–e280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeld, R.M. Acute Sinusitis in Adults. N. Engl. J. Med. 2016, 375, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Gwaltney, J.M.; Scheld, W.M.; Sande, M.A.; Sydnor, A. The Microbial Etiology and Antimicrobial Therapy of Adults with Acute Community-Acquired Sinusitis: A Fifteen-Year Experience at the University of Virginia and Review of Other Selected Studies. J. Allergy Clin. Immunol. 1992, 90, 457–462. [Google Scholar] [CrossRef]
- Şentürk, M. Medical Management of Paranasal Sinus Infection. In Challenging Issues on Paranasal Sinuses; IntechOpen: London, UK, 2018; pp. 57–78. [Google Scholar]
- Patel, Z.M.; Hwang, P.H. Acute Bacterial Rhinosinusitis. In Infections of the Ears, Nose, Throat, and Sinuses; Durand, M., Deschler, D., Eds.; Springer International Publishing AG, part of Springer Nature: New York, NY, USA, 2018; pp. 1–143. ISBN 9783319748351. [Google Scholar]
- Scheid, D.C.; Hamm, R.M. Acute Bacterial Rhinosinusitis in Adults: Part I. Evaluation. Am. Fam. Physician 2004, 70, 1685–1692. [Google Scholar] [PubMed]
- Homayun, B.; Lin, X.; Choi, H. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemiengre, M.; van Driel, M.; Merestein, D.; Liira, H.; Makela, M.; De Sutter, A.I. Antibiotics for Acute Rhinosinusitis in Adults (Review). Cochrane Database Syst. Rev. 2018, 2018, 1–80. [Google Scholar] [CrossRef] [Green Version]
- Axiotakis, L.G.; Szeto, B.; Gonzalez, J.N.; Caruana, F.F.; Gudis, D.A.; Overdevest, J.B. Antibiotic Adverse Effects in Pediatric Acute Rhinosinusitis: Systematic Review and Meta-Analysis. Int. J. Pediatr. Otorhinolaryngol. 2022, 156, 111064. [Google Scholar] [CrossRef]
- De Velde, F.; De Winter, B.C.M.; Koch, B.C.P.; Van Gelder, T.; Mouton, J.W. Non-Linear Absorption Pharmacokinetics of Amoxicillin: Consequences for Dosing Regimens and Clinical Breakpoints. J. Antimicrob. Chemother. 2016, 71, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral Amoxicillin and Amoxicillin–Clavulanic Acid: Properties, Indications and Usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2021, 12, 735–757. [Google Scholar] [CrossRef]
- Martin, V.; Hoekman, J.; Aurora, S.K.; Shrewsbury, S.B. Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. J. Clin. Med. 2021, 10, 2468. [Google Scholar] [CrossRef]
- Cingi, C.; Bayar Muluk, N.; Mitsias, D.I.; Papadopoulos, N.G.; Klimek, L.; Laulajainen-Hongisto, A.; Hytönen, M.; Toppila-Salmi, S.K.; Scadding, G.K. The Nose as a Route for Therapy: Part 1. Pharmacotherapy. Front. Allergy 2021, 2, 638136. [Google Scholar] [CrossRef]
- Beule, A.G. Physiology and Pathophysiology of Respiratory Mucosa of the Nose and the Paranasal Sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, 1–24. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, J.; Alves, G.; Oliveira, P.; Fortuna, A.; Falcão, A. Intranasal delivery of ciprofloxacin to rats: A topical approach using a thermoreversible in situ gel. Eur. J. Pharm. Sci. 2017, 97, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Fatouros, D.G. Smart Materials: In Situ Gel-Forming Systems for Nasal Delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-Responsive In Situ Gelling System for Nose-to-Brain Drug Delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In Situ-Based Gels for Nose to Brain Delivery for the Treatment of Neurological Diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Chonkar, A.; Nayak, U.; Udupa, N. Smart Polymers in Nasal Drug Delivery. Indian J. Pharm. Sci. 2015, 77, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lee, J.; Meng, M.; Cui, N.; Dai, C.Y.; Jia, Q.; Lee, E.; Chen, Y.; Chen, Y.; Lee, J.; et al. An Overview on Thermosensitive Oral Gel Based on An Overview on Thermosensitive Oral Gel Based on Poloxamer. Materials 2021, 14, 4522. [Google Scholar] [CrossRef]
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive Polymers: Insights into Decisive Hydrogel Characteristics, Mechanisms of Gelation, and Promising Biomedical Applications. Int. J. Pharm. 2014, 472, 262–275. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.; Ansa, C.R.; Cinu, T.A.; Chacko, A.J.; Aleykutty, N.A.; Ferreira, S.V.; Souto, E.B. Thermo-Sensitive Gels Containing Lorazepam Microspheres for Intranasal Brain Targeting. Int. J. Pharm. 2013, 441, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Jelvehgari, M. Thermosensitive in Situ Nanocomposite of Rivastigmine Hydrogen Tartrate as an Intranasal Delivery System: Development, Characterization, Ex Vivo Permeation and Cellular Studies. Colloids Surf. B Biointerfaces 2017, 159, 629–638. [Google Scholar] [CrossRef]
- Sridhar, V.; Wairkar, S.; Gaud, R.; Bajaj, A.; Meshram, P. Brain Targeted Delivery of Mucoadhesive Thermosensitive Nasal Gel of Selegiline Hydrochloride for Treatment of Parkinson’s Disease. J. Drug Target. 2018, 26, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Abouhussein, D.M.N.; Khattab, A.; Bayoumi, N.A.; Mahmoud, A.F.; Sakr, T.M. Brain Targeted Rivastigmine Mucoadhesive Thermosensitive In Situ Gel: Optimization, in Vitro Evaluation, Radiolabeling, in Vivo Pharmacokinetics and Biodistribution. J. Drug Deliv. Sci. Technol. 2018, 43, 129–140. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H. Intranasal Optimized Solid Lipid Nanoparticles Loaded in Situ Gel for Enhancing Trans-Mucosal Delivery of Simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Katona, G.; Sipos, B.; Budai-Szűcs, M.; Balogh, G.T.; Veszelka, S.; Gróf, I.; Deli, M.A.; Volk, B.; Szabó-Révész, P.; Csóka, I. Development of in Situ Gelling Meloxicam-Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application. Pharmaceutics 2021, 13, 646. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-Based Intranasal Thermoreversible Gel of Zolmitriptan-Loaded Nanoethosomes: Formulation, Optimization, Evaluation and Permeation Studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.A.H.A.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; EL-Massik, M.A.E.; Boraie, N.A. A Novel Nasal Almotriptan Loaded Solid Lipid Nanoparticles in Mucoadhesive in Situ Gel Formulation for Brain Targeting: Preparation, Characterization and in Vivo Evaluation. Int. J. Pharm. 2018, 548, 609–624. [Google Scholar] [CrossRef]
- Adnet, T.; Groo, A.C.; Picard, C.; Davis, A.; Corvaisier, S.; Since, M.; Bounoure, F.; Rochais, C.; Le Pluart, L.; Dallemagne, P.; et al. Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer’s Disease Treatment. Pharmaceutics 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In Situ Gelling and Mucoadhesive Polymers: Why Do They Need Each Other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.G.; Azoia, N.C.; Gomes, A.; Cavaco-Paulo, A. Albumin-Based Nanodevices as Drug Carriers. Curr. Pharm. Des. 2016, 22, 1371–1390. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-Based Nanoparticles as Potential Controlled Release Drug Delivery Systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhao, X.; Zu, Y.; Li, J.; Zhang, Y.; Jiang, R.; Zhang, Z. Preparation, Characterization, and in Vitro Targeted Delivery of Folate-Decorated Paclitaxel-Loaded Bovine Serum Albumin Nanoparticles. Int. J. Nanomed. 2010, 5, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; He, X.Y.; Liu, B.Y.; Xu, C.; Ai, S.L.; Zhuo, R.X.; Cheng, S.X. Aptamer-Functionalized Albumin-Based Nanoparticles for Targeted Drug Delivery. Colloids Surf. B Biointerfaces 2018, 171, 24–30. [Google Scholar] [CrossRef]
- Karami, K.; Jamshidian, N.; Hajiaghasi, A.; Amirghofran, Z. BSA Nanoparticles as Controlled Release Carriers for Isophethalaldoxime Palladacycle Complex; Synthesis, Characterization: In Vitro Evaluation, Cytotoxicity and Release Kinetics Analysis. New J. Chem. 2020, 44, 4394–4405. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, K.; Patel, S. Bovine Serum Albumin Nanoparticles for the Efficient Delivery of Berberine: Preparation, Characterization and In Vitro Biological Studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125501. [Google Scholar] [CrossRef]
- Boyuklieva, R.; Pilicheva, B. Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines 2022, 10, 1706. [Google Scholar] [CrossRef]
- Arabi, S.H.; Aghelnejad, B.; Schwieger, C.; Meister, A.; Kerth, A.; Hinderberger, D. Serum Albumin Hydrogels in Broad PH and Temperature Ranges: Characterization of Their Self-Assembled Structures and Nanoscopic and Macroscopic Properties. Biomater. Sci. 2018, 6, 478–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katona, G.; Sipos, B.; Csóka, I. Risk-Assessment-Based Optimization Favours the Development of Albumin Nanoparticles with Proper Characteristics Prior to Drug Loading. Pharmaceutics 2022, 14, 2036. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Figueroa, M.J.; Alarcón, D.A.; González-Aramúndiz, J.V. Effect of Zeta Potential of Innovative Lipid Nanocapsules on Triamcinolone Transdermal Delivery. Drug Deliv. Transl. Res. 2022, 12, 2740–2750. [Google Scholar] [CrossRef]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of Surface Charge on the Brain Delivery of Nanostructured Lipid Carriers in Situ Gels via the Nasal Route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef]
- Amighi, F.; Emam-Djomeh, Z.; Labbafi-Mazraeh-Shahi, M. Effect of Different Cross-Linking Agents on the Preparation of Bovine Serum Albumin Nanoparticles. J. Iran. Chem. Soc. 2020, 17, 1223–1235. [Google Scholar] [CrossRef]
- Jones, N. The Nose and Paranasal Sinuses Physiology and Anatomy. Adv. Drug Deliv. Rev. 2001, 51, 5–19. [Google Scholar] [CrossRef]
- Russo, J.; Fiegel, J.; Brogden, N.K. Rheological and drug delivery characteristics of poloxamer-based diclofenac sodium formulations for chronic wound site analgesia. Pharmaceutics 2020, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A Mucoadhesive, Thermoreversible in Situ Nasal Gel of Geniposide for Neurodegenerative Diseases. PLoS ONE 2017, 12, e0189478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verekar, R.R.; Gurav, S.S.; Bolmal, U. Thermosensitive Mucoadhesive in Situ Gel for Intranasal Delivery of Almotriptan Malate: Formulation, Characterization, and Evaluation. J. Drug Deliv. Sci. Technol. 2020, 58, 101778. [Google Scholar] [CrossRef]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Rheological, Adhesive and Release Characterisation of Semisolid Carbopol/Tetraglycol Systems. Int. J. Pharm. 2006, 307, 129–140. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Qian, S.; Wong, Y.C.; Zuo, Z. Development, Characterization and Application of in Situ Gel Systems for Intranasal Delivery of Tacrine. Int. J. Pharm. 2014, 468, 272–282. [Google Scholar] [CrossRef]
- Yong, C.S.; Choi, J.S.; Quan, Q.Z.; Rhee, J.D.; Kim, C.K.; Lim, S.J.; Kim, K.M.; Oh, P.S.; Choi, H.G. Effect of Sodium Chloride on the Gelation Temperature, Gel Strength and Bioadhesive Force of Poloxamer Gels Containing Diclofenac Sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef]
- Ferreira, N.N.; de Oliveira Junior, E.; Granja, S.; Boni, F.I.; Ferreira, L.M.B.; Cury, B.S.F.; Santos, L.C.R.; Reis, R.M.; Lima, E.M.; Baltazar, F.; et al. Nose-to-Brain Co-Delivery of Drugs for Glioblastoma Treatment Using Nanostructured System. Int. J. Pharm. 2021, 603, 120714. [Google Scholar] [CrossRef]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in Brain Targeting Drug Delivery System by Nasal Route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal Delivery of Nanostructured Lipid Carriers, Solid Lipid Nanoparticles and Nanoemulsions: A Current Overview of in Vivo Studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for Direct Nose-to-Brain Delivery of Drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Huang, T.H.; Yang, S.C.; Chen, C.C.; Fang, J.Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (ZnO Nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Aruguete, D.M.; Hochella, M.F. Bacteria-Nanoparticle Interactions and Their Environmental Implications. Environ. Chem. 2010, 7, 3–9. [Google Scholar] [CrossRef]
- Bastier, P.L.; Lechot, A.; Bordenave, L.; Durand, M.; De Gabory, L. Nasal Irrigation: From Empiricism to Evidence-Based Medicine. A Review. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, C.K.; Dulfano, M.J. Effect of PH, Viscosity and Ionic-Strength Changes on Ciliary Beating Frequency of Human Bronchial Explants. Clin. Sci. 1983, 64, 449–451. [Google Scholar] [CrossRef] [Green Version]
- Brambilla, E.; Locarno, S.; Gallo, S.; Orsini, F.; Pini, C.; Farronato, M.; Thomaz, D.V.; Lenardi, C.; Piazzoni, M.; Tartaglia, G. Poloxamer-Based Hydrogel as Drug Delivery System: How Polymeric Excipients Influence the Chemical-Physical Properties. Polymers 2022, 14, 3624. [Google Scholar] [CrossRef]
- England, R.J.A.; Homer, J.J.; Knight, L.C.; Ell, S.R. Nasal PH Measurement: A Reliable and Repeatable Parameter. Clin. Otolaryngol. Allied Sci. 1999, 24, 67–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washington, N.; Steele, R.J.C.; Jackson, S.J.; Bush, D.; Mason, J.; Gill, D.A.; Pitt, K.; Rawlins, D.A. Determination of Baseline Human Nasal PH and the Effect of Intranasally Administered Buffers. Int. J. Pharm. 2000, 198, 139–146. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, J.H.; Kim, S.W.; Kim, S.W.; Jin, K.S.; Cho, J.H.; Kang, J.M.; Park, S.Y. Nasal PH in Patients with Chronic Rhinosinusitis before and after Endoscopic Sinus Surgery. Am. J. Otolaryngol. Head Neck Med. Surg. 2013, 34, 505–507. [Google Scholar] [CrossRef]
- Kumar, R.A.; Viswanatha, B.; Krishnamurthy, N.; Jayanna, N.; Shetty, D.R. Efficacy of Hypertonic Saline and Normal Saline in the Treatment of Chronic Sinusitis. Int. J. Otolaryngol. Head & Neck Surg. 2013, 02, 90–96. [Google Scholar] [CrossRef]
- Rabago, D.; Pasic, T.; Zgierska, A.; Mundt, M.; Barrett, B.; Maberry, R. The Efficacy of Hypertonic Saline Nasal Irrigation for Chronic Sinonasal Symptoms. Otolaryngol. Head Neck Surg. 2005, 133, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, M.; Li, Y.; Tan, G.; Yang, Y. Efficacy of Nasal Irrigation with Hypertonic Saline on Chronic Rhinosinusitis: Systematic Review and Meta-Analysis. Braz. J. Otorhinolaryngol. 2020, 86, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Moffa, A.; Cassano, M.; Carinci, F.; Lopez, M.A.; Trecca, E.M.C.; Torretta, S.; Rinaldi, V.; Pignataro, L. Saline Nasal Irrigations for Chronic Rhinosinusitis: From Everyday Practice to Evidence-Based Medicine. An Update. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418802676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culig, J.; Leppée, M.; Vceva, A.; Djanic, D. Efficiency of Hypertonic and Isotonic Seawater Solutions in Chronic Rhinosinusitis. Med. Glas. 2010, 7, 116–123. [Google Scholar]
- Friedman, M.; Vidyasagar, R.; Joseph, N. A Randomized, Prospective, Double-Blind Study on the Efficacy of Dead Sea Salt Nasal Irrigation. Laryngoscope 2006, 116, 878–882. [Google Scholar] [CrossRef]
- Kanjanawasee, D.; Seresirikachorn, K.; Chitsuthipakorn, W.; Snidvongs, K. Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2018, 32, 269–279. [Google Scholar] [CrossRef]
- Rabago, D.; Zgierska, A.; Mundt, M.; Barrett, B.; Bobula, J.; Maberry, R. Efficacy of Daily Hypertonic Saline Nasal Irrigation among Patients with Sinusitis: A Randomized Controlled Trial. J. Fam. Pract. 2002, 51, 1049–1055. [Google Scholar]
- Shoseyov, D.; Bibi, H.; Shai, P.; Shoseyov, N.; Shazberg, G.; Hurvitz, H. Treatment with Hypertonic Saline versus Normal Saline Nasal Wash of Pediatric Chronic Sinusitis. J. Allergy Clin. Immunol. 1998, 101, 602–605. [Google Scholar] [CrossRef]
- Rabago, D.; Barrett, B.; Marchand, L.; Maberry, R.; Mundt, M. Qualitative Aspects of Nasal Irrigation Use by Patients with Chronic Sinus Disease in a Multimethod Study. Ann. Fam. Med. 2006, 4, 295–301. [Google Scholar] [CrossRef]
- Moreno-Bautista, G.; Tam, K.C. Evaluation of Dialysis Membrane Process for Quantifying the in Vitro Drug-Release from Colloidal Drug Carriers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 299–303. [Google Scholar] [CrossRef]
- Akkari, A.C.S.; Papini, J.Z.B.; Garcia, G.K.; Franco, M.K.K.D.; Cavalcanti, L.P.; Gasperini, A.; Alkschbirs, M.I.; Yokaichyia, F.; De Paula, E.; Tófoli, G.R.; et al. Poloxamer 407/188 Binary Thermosensitive Hydrogels as Delivery Systems for Infiltrative Local Anesthesia: Physico-Chemical Characterization and Pharmacological Evaluation. Mater. Sci. Eng. C 2016, 68, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST Disk Diffusion Antimicrobial Susceptibility Testing Method and Its Implementation in Routine Microbiology Laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
- The European Committe on Antimicrobial Susceptibility Testing (EUCAST). Disk Diffusion Method for Antimicrobial Susceptibility Testing Version 10.0. 2022. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 27 August 2022).
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Gao, Y.; Jin, Q.; Ji, J. Emerging Antibacterial Nanomedicine For Enhanced Antibiotic Therapy. Biomater. Sci. 2020, 8, 6825–6839. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent Advances in the Development of in Situ Gelling Drug Delivery Systems for Non-Parenteral Administration Routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef]
- Voumard, R.; Van Neyghem, N.; Cochet, C.; Gardiol, C.; Decosterd, L.; Buclin, T.; de Valliere, S. Antibiotic Stability Related to Temperature Variations in Elastomeric Pumps Used for Outpatient Parenteral Antimicrobial Therapy (OPAT). J. Antimicrob. Chemother. 2017, 72, 1462–1465. [Google Scholar] [CrossRef] [Green Version]
- Walker, S.E.; Iazzetta, J.; Law, S.; Biniecki, K. Stability of Commonly Used Antibiotic Solutions in an Elastomeric Infusion Device. Can. J. Hosp. Pharm. 2010, 63, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Samara, E.; Moriarty, T.; Richards, R.; Decosterd, L.; Gautier, E.; Wahl, P. Antibiotic Stability over Six Weeks in Aqueous Solution at Body Tmperature with and without Heat Treatment That Mimics the Curing Body Cement. Bone Jt. J. 2017, 6, 296–306. [Google Scholar] [CrossRef]
- Purohit, T.J.; Wu, Z.; Hanning, S.M. Simple and Reliable Extraction and a Validated High Performance Liquid Chromatographic Assay for Quantification of Amoxicillin from Plasma. J. Chromatogr. A 2020, 1611, 460611. [Google Scholar] [CrossRef]
- Arabi, S.H.; Haselberger, D.; Hinderberger, D. The effect of ethanol on gelation, nanoscopic, and macroscopic properties of serum albumin hydrogels. Molecules 2020, 25, 1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipos, B.; Budai-Szűcs, M.; Kókai, D.; Orosz, L.; Burián, K.; Csorba, A.; Nagy, Z.Z.; Balogh, G.T.; Csóka, I.; Katona, G. Erythromycin-loaded polymeric micelles: In situ gel development, in vitro and ex vivo ocular investigations. Eur. J. Pharm. Biopharm. 2022, 180, 81–90. [Google Scholar] [CrossRef] [PubMed]









| Standard Run | Independent Variables | Z-Average ± SD (nm) | PdI ± SD | ZP ± SD (mV) | ||
|---|---|---|---|---|---|---|
| AMT (mg) | PW (mL) | Ethanol (mL) | ||||
| 1 | 5.0 | 0.9 | 0.8 | 69.37 ± 3.2 | 0.603 ± 0.03 | −27.4 ± 0.1 |
| 2 | 5.0 | 1.0 | 1.0 | 140.2 ± 2.1 | 0.498 ±.0.01 | −32.1 ± 0.4 |
| 3 | 5.0 | 1.1 | 0.9 | 61.43 ± 3.5 | 0.582 ±0.03 | −24.9 ± 0.2 |
| 4 | 7.5 | 0.9 | 1.0 | 239.4 ± 1.9 | 0.498 ±0.02 | −33.1 ± 0.3 |
| 5 | 7.5 | 1.0 | 0.9 | 163.2 ± 4.6 | 0.522 ±0.04 | −29.4 ± 0.5 |
| 6 | 7.5 | 1.1 | 0.8 | 18.18 ± 2.5 | 0.825 ±0.02 | −20.5 ± 0.1 |
| 7 | 2.5 | 0.9 | 0.9 | 240.9 ± 2.3 | 0.629 ±0.05 | −36.8 ± 0.4 |
| 8 | 2.5 | 1.0 | 0.8 | 103.1 ± 3.1 | 0.495 ± 0.03 | −32.2 ± 0.2 |
| 9 | 2.5 | 1.1 | 1.0 | 115.8 ± 2.6 | 0.493 ± 0.01 | −33.3 ± 0.1 |
| Formulation | Z-Average ± SD (nm) | PdI ± SD | Zeta Potential ± SD (mV) |
|---|---|---|---|
| AMT-BSA | 71.32 ± 1.73 | 0.593 ± 0.01 | −29.3667 ± 0.4 |
| 21% w/v | 118.43 ± 3.02 | 0.615 ± 0.02 | −18.4333 ± 0.3 |
| 22% w/v | 134.53 ± 2.90 | 0.648 ± 0.03 | −18.6667 ± 0.1 |
| 23% w/v | 137.20 ± 2.66 | 0.682 ± 0.01 | −19.7667 ± 0.5 |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| AMT (mg) | 2.5 | 5.0 | 7.5 |
| PW (mL) | 0.9 | 1.0 | 1.1 |
| Ethanol (mL) | 0.8 | 0.9 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardikasari, S.A.; Budai-Szűcs, M.; Orosz, L.; Burián, K.; Csóka, I.; Katona, G. Development of Thermoresponsive-Gel-Matrix-Embedded Amoxicillin Trihydrate-Loaded Bovine Serum Albumin Nanoparticles for Local Intranasal Therapy. Gels 2022, 8, 750. https://doi.org/10.3390/gels8110750
Mardikasari SA, Budai-Szűcs M, Orosz L, Burián K, Csóka I, Katona G. Development of Thermoresponsive-Gel-Matrix-Embedded Amoxicillin Trihydrate-Loaded Bovine Serum Albumin Nanoparticles for Local Intranasal Therapy. Gels. 2022; 8(11):750. https://doi.org/10.3390/gels8110750
Chicago/Turabian StyleMardikasari, Sandra Aulia, Mária Budai-Szűcs, László Orosz, Katalin Burián, Ildikó Csóka, and Gábor Katona. 2022. "Development of Thermoresponsive-Gel-Matrix-Embedded Amoxicillin Trihydrate-Loaded Bovine Serum Albumin Nanoparticles for Local Intranasal Therapy" Gels 8, no. 11: 750. https://doi.org/10.3390/gels8110750
APA StyleMardikasari, S. A., Budai-Szűcs, M., Orosz, L., Burián, K., Csóka, I., & Katona, G. (2022). Development of Thermoresponsive-Gel-Matrix-Embedded Amoxicillin Trihydrate-Loaded Bovine Serum Albumin Nanoparticles for Local Intranasal Therapy. Gels, 8(11), 750. https://doi.org/10.3390/gels8110750