Three-Dimensional Bio-Printed Cardiac Patch for Sustained Delivery of Extracellular Vesicles from the Interface
Abstract
:1. Introduction
2. Results
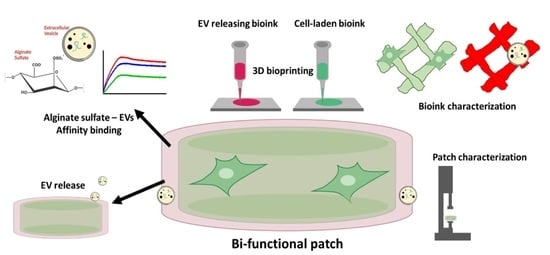
2.1. Cardiac Patch Design
2.2. Rheological Properties and 3D Bioprinting of Alginate-Based Solutions
2.2.1. Cell-Laden Core Bioink Solutions
2.2.2. Core 3D Bio-Printing Optimization
2.2.3. Shell Bioink Solutions
2.3. Two-Component Cardiac Patch Fabrication
2.4. EVs Interacts with Alginate Sulfate through Affinity-Binding
2.5. Three-Dimensional Bio-Printing of AlgS-EVs Complexes Encapsulated within Alginate Hydrogel
2.6. Alginate Sulfate Prolongs EV Release Profile from 3D-Printed Alginate Matrix
3. Discussion
3.1. Cardiac Patch Design and 3D Bio-Printing of Inner Core
3.2. Three-Dimensional Bio-Printing of Outer Shell and Alginate Sulfate—EVs Delivery System
3.3. Research Outlook and Future Prespective
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Cell Culture
5.3. Extracellular Vesicles Isolation and Characterization
5.3.1. Extracellular Vesicles Isolation
5.3.2. Nanoparticle Tracking Analysis (NTA) of Size and Concentration
5.4. Surface Plasmon Resonance (SPR) Analysis of the Molecular Interactions of Alginate Sulfate with EVs
5.5. Three-Dimensional Bio-Printing
5.5.1. Shell Bioink Solution Preparation
5.5.2. Core Bioink Solution Preparation
5.5.3. Rheological Characterization of Bioink Solutions
5.5.4. Three-Dimensional Bio-Printing Procedure and Constructs Maintenance
5.5.5. Printability Analysis
5.6. Cryogenic Scanning Electron Microscopy (Cryo-SEM)
5.7. Mechanical Stiffness of 3D Bio-Printed Cardiac Constructs
5.8. EVs Release Studies
5.8.1. Preparation of Alginate/Alginate-Sulfate Hydrogel Microspheres with EVs
5.8.2. Release from 3D Bio-Printed Constructs
5.9. Cell Metabolic Activity and DNA Content
5.10. Immunostaining and Confocal Imaging
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Laflamme, M.A.; Murry, C.E. Heart Regeneration. Nature 2011, 473, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laflamme, M.A.; Murry, C.E. Regenerating the Heart. Nat. Biotechnol. 2005, 23, 845. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.; Langer, R.; Vacanti, J. (Eds.) Principles of Tissue Engineering, 4th ed.; Academic Press: Boston, MA, USA, 2014; p. iv. ISBN 978-0-12-398358-9. [Google Scholar]
- Menasché, P. Current Status and Future Prospects for Cell Transplantation to Prevent Congestive Heart Failure. Semin. Thorac. Cardiovasc. Surg. 2008, 20, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, R.J.; Maillet, M.; Sargent, M.A.; Khalil, H.; Johansen, A.K.; Schwanekamp, J.A.; York, A.J.; Huang, V.; Nahrendorf, M.; Sadayappan, S.; et al. An Acute Immune Response Underlies the Benefit of Cardiac Stem-Cell Therapy. Nature 2019, 577, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Halushka, M.K. Extracellular Vesicle MicroRNA Transfer in Cardiovascular Disease. Cardiovasc. Pathol. 2015, 24, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Raposo, G. Exosomes–Vesicular Carriers for Intercellular Communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.; Steadman, R.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer Exosomes Trigger Fibroblast to Myofibroblast Differentiation. Cancer Res. 2010, 70, 9621–9630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat. Cell Biol. 2015, 17, 816. [Google Scholar] [CrossRef]
- Emanueli, C.; Shearn, A.I.U.; Angelini, G.D.; Sahoo, S. Exosomes and Exosomal MiRNAs in Cardiovascular Protection and Repair. Vascul. Pharmacol. 2015, 71, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, D.; Chen, X.; Li, J.; Li, L.; Bian, Z.; Sun, F.; Lu, J.; Yin, Y.; Cai, X. Secreted Monocytic MiR-150 Enhances Targeted Endothelial Cell Migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.E.; Timmers, L.; Van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K/Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, S.W.; Wang, J.; Lee, C.J.; Liu, M.; Neelamegham, S.; Canty, J.M.; Nguyen, J. The MicroRNA Regulatory Landscape of MSC-Derived Exosomes: A Systems View. Sci. Rep. 2018, 8, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.H.; Chen, W.; Shao, N.; Xiao, D.; Qin, X.; Baker, N.; Bae, H.R.; Wei, T.; Wang, Y.; Shukla, P. Comparison of Non-coding RNAs in Exosomes and Functional Efficacy of Human Embryonic Stem Cell-versus Induced Pluripotent Stem Cell-derived Cardiomyocytes. Stem Cells 2017, 35, 2138–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishore, R.; Khan, M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ. Res. 2016, 118, 330–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2016, 45, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tibbitt, M.W.; Langer, R. Living Biomaterials. Acc. Chem. Res. 2017, 50, 508–513. [Google Scholar] [CrossRef]
- Wong, P.T.; Choi, S.K. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef]
- Liu, R.; Wu, Q.; Huang, X.; Zhao, X.; Chen, X.; Chen, Y.; Weitz, D.A.; Song, Y. Synthesis of Nanomedicine Hydrogel Microcapsules by Droplet Microfluidic Process and Their PH and Temperature Dependent Release. RSC Adv. 2021, 11, 37814–37823. [Google Scholar] [CrossRef]
- Patel, B.; Manne, R.; Patel, D.B.; Gorityala, S.; Palaniappan, A.; Kurakula, M. Chitosan as Functional Biomaterial for Designing Delivery Systems in Cardiac Therapies. Gels 2021, 7, 253. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruvinov, E.; Cohen, S. Alginate Biomaterial for the Treatment of Myocardial Infarction: Progress, Translational Strategies, and Clinical Outlook: From Ocean Algae to Patient Bedside. Adv. Drug Deliv. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Follin, B.; Juhl, M.; Cohen, S.; Pedersen, A.E.; Gad, M.; Kastrup, J.; Ekblond, A. Human Adipose-Derived Stromal Cells in a Clinically Applicable Injectable Alginate Hydrogel: Phenotypic and Immunomodulatory Evaluation. Cytotherapy 2015, 17, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Leor, J.; Tuvia, S.; Guetta, V.; Manczur, F.; Castel, D.; Willenz, U.; Petneházy, Ö.; Landa, N.; Feinberg, M.S.; Konen, E. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. J. Am. Coll. Cardiol. 2009, 54, 1014–1023. [Google Scholar] [CrossRef] [Green Version]
- Re’em, T.; Witte, F.; Willbold, E.; Ruvinov, E.; Cohen, S. Simultaneous Regeneration of Articular Cartilage and Subchondral Bone Induced by Spatially Presented TGF-Beta and BMP-4 in a Bilayer Affinity Binding System. Acta Biomater. 2012, 8, 3283–3293. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/Chitosan/Alginate Ternary Scaffolds for Cardiac Tissue Engineering Application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Sondermeijer, H.P.; Witkowski, P.; Seki, T.; Van der Laarse, A.; Itescu, S.; Hardy, M.A. RGDfK-Peptide Modified Alginate Scaffold for Cell Transplantation and Cardiac Neovascularization. Tissue Eng. Part A 2018, 24, 740–751. [Google Scholar] [CrossRef]
- Sapir, Y.; Cohen, S.; Friedman, G.; Polyak, B. The Promotion of in Vitro Vessel-like Organization of Endothelial Cells in Magnetically Responsive Alginate Scaffolds. Biomaterials 2012, 33, 4100–4109. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, J.K.; Opara, E.C.; Brey, E.M. Alginate-Based Strategies for Therapeutic Vascularization. Ther. Deliv. 2013, 4, 327–341. [Google Scholar] [CrossRef]
- Freeman, I.; Kedem, A.; Cohen, S. The Effect of Sulfation of Alginate Hydrogels on the Specific Binding and Controlled Release of Heparin-Binding Proteins. Biomaterials 2008, 29, 3260–3268. [Google Scholar] [CrossRef]
- Re’em, T.; Kaminer-Israeli, Y.; Ruvinov, E.; Cohen, S. Chondrogenesis of HMSC in Affinity-Bound TGF-Beta Scaffolds. Biomaterials 2012, 33, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Ruvinov, E.; Leor, J.; Cohen, S. The Promotion of Myocardial Repair by the Sequential Delivery of IGF-1 and HGF from an Injectable Alginate Biomaterial in a Model of Acute Myocardial Infarction. Biomaterials 2011, 32, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Masasa, H.; Orenbuch, A.; Levaot, N.; Goldenberg, M.S.; Cohen, S. Reconstruction of the Ovary Microenvironment Utilizing Macroporous Scaffold with Affinity-Bound Growth Factors. Biomaterials 2019, 205, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.-Q.; Snodgrass, H.R.; Bursac, N. Tissue-Engineered Cardiac Patch for Advanced Functional Maturation of Human ESC-Derived Cardiomyocytes. Biomaterials 2013, 34, 5813–5820. [Google Scholar] [CrossRef] [Green Version]
- Liau, B.; Christoforou, N.; Leong, K.W.; Bursac, N. Pluripotent Stem Cell-Derived Cardiac Tissue Patch with Advanced Structure and Function. Biomaterials 2011, 32, 9180–9187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.-L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- Jabbour, R.J.; Owen, T.J.; Pandey, P.; Reinsch, M.; Wang, B.; King, O.; Couch, L.S.; Pantou, D.; Pitcher, D.S.; Chowdhury, R.A. In Vivo Grafting of Large Engineered Heart Tissue Patches for Cardiac Repair. JCI Insight 2021, 6, 15. [Google Scholar] [CrossRef]
- Park, B.-W.; Jung, S.-H.; Das, S.; Lee, S.M.; Park, J.-H.; Kim, H.; Hwang, J.-W.; Lee, S.; Kim, H.-J.; Kim, H.-Y. In Vivo Priming of Human Mesenchymal Stem Cells with Hepatocyte Growth Factor–Engineered Mesenchymal Stem Cells Promotes Therapeutic Potential for Cardiac Repair. Sci. Adv. 2020, 6, eaay6994. [Google Scholar] [CrossRef] [Green Version]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D. A Multi-Cellular 3D Bioprinting Approach for Vascularized Heart Tissue Engineering Based on HUVECs and IPSC-Derived Cardiomyocytes. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Ajdary, R.; Ezazi, N.Z.; Correia, A.; Kemell, M.; Huan, S.; Ruskoaho, H.J.; Hirvonen, J.; Santos, H.A.; Rojas, O.J. Multifunctional 3D-printed Patches for Long-term Drug Release Therapies after Myocardial Infarction. Adv. Funct. Mater. 2020, 30, 2003440. [Google Scholar] [CrossRef]
- Sachlos, E.; Czernuszka, J.T. Making Tissue Engineering Scaffolds Work. Review: The Application of Solid Freeform Fabrication Technology to the Production of Tissue Engineering Scaffolds. Eur. Cell. Mater. 2003, 5, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Muschler, G.F.; Nakamoto, C.; Griffith, L.G. Engineering Principles of Clinical Cell-Based Tissue Engineering. JBJS 2004, 86, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-S.V.; Kim, C.; Mercola, M. Electrophysiological Challenges of Cell-Based Myocardial Repair. Circulation 2009, 120, 2496–2508. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human Embryonic-Stem-Cell-Derived Cardiomyocytes Regenerate Non-Human Primate Hearts. Nature 2014, 510, 273. [Google Scholar] [CrossRef] [Green Version]
- Jackman, C.P.; Ganapathi, A.M.; Asfour, H.; Qian, Y.; Allen, B.W.; Li, Y.; Bursac, N. Engineered Cardiac Tissue Patch Maintains Structural and Electrical Properties after Epicardial Implantation. Biomaterials 2018, 159, 48–58. [Google Scholar] [CrossRef]
- Wang, J.; Chu, R.; Ni, N.; Nan, G. The Effect of Matrigel as Scaffold Material for Neural Stem Cell Transplantation for Treating Spinal Cord Injury. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.H.; Kim, K.S.; Park, S.H.; Suh, Y.S.; Kim, S.J.; Jeun, S.S.; Sung, Y.C. The Effects of Mesenchymal Stem Cells Injected via Different Routes on Modified IL-12-Mediated Antitumor Activity. Gene Ther. 2011, 18, 488–495. [Google Scholar] [CrossRef]
- Annamalai, B.; Parsons, N.; Brandon, C.; Rohrer, B. The Use of Matrigel Combined with Encapsulated Cell Technology to Deliver a Complement Inhibitor in a Mouse Model of Choroidal Neovascularization. Mol. Vis. 2020, 26, 370. [Google Scholar]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic Alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Atai, N.A.; Chen, W.; Mu, D.; Tannous, B.A.; Breakefield, X.O.; Skog, J.; Maguire, C.A. Heparin Affinity Purification of Extracellular Vesicles. Sci. Rep. 2015, 5, 10266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, I.; Cohen, S. The Influence of the Sequential Delivery of Angiogenic Factors from Affinity-Binding Alginate Scaffolds on Vascularization. Biomaterials 2009, 30, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.D.; Landázuri, N.; Phelps, E.A.; Brown, M.E.; García, A.J.; Davis, M.E.; Joseph, G.; Long, R., Jr.; Safley, S.A.; Suever, J.D. Cellular Encapsulation Enhances Cardiac Repair. J. Am. Heart Assoc. 2013, 2, e000367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, E.T.; Hastings, C.L.; Lewin, S.A.; Shvartsman, D.E.; Brudno, Y.; Vasilyev, N.V.; O’Brien, F.J.; Walsh, C.J.; Duffy, G.P.; Mooney, D.J. Comparison of Biomaterial Delivery Vehicles for Improving Acute Retention of Stem Cells in the Infarcted Heart. Biomaterials 2014, 35, 6850–6858. [Google Scholar] [CrossRef] [Green Version]
- Tsur-Gang, O.; Ruvinov, E.; Landa, N.; Holbova, R.; Feinberg, M.S.; Leor, J.; Cohen, S. The Effects of Peptide-Based Modification of Alginate on Left Ventricular Remodeling and Function after Myocardial Infarction. Biomaterials 2009, 30, 189–195. [Google Scholar] [CrossRef]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and Cell Viability in Bioprinting Alginate Dialdehyde-Gelatin Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef]
- Giorleo, L.; Tegazzini, F.; Sartore, L. 3D Printing of Gelatin/Chitosan Biodegradable Hybrid Hydrogel: Critical Issues Due to the Crosslinking Reaction, Degradation Phenomena and Process Parameters. Bioprinting 2021, 24, e00170. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D Printing of Gelatin Methacrylamide Cell-Laden Tissue-Engineered Constructs with High Cell Viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting Is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Sapir, Y.; Kryukov, O.; Cohen, S. Integration of Multiple Cell-Matrix Interactions into Alginate Scaffolds for Promoting Cardiac Tissue Regeneration. Biomaterials 2011, 32, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Hayoun-Neeman, D.; Korover, N.; Etzion, S.; Ofir, R.; Lichtenstein, R.G.; Cohen, S. Exploring Peptide-Functionalized Alginate Scaffolds for Engineering Cardiac Tissue from Human Embryonic Stem Cell-Derived Cardiomyocytes in Serum-Free Medium. Polym. Adv. Technol. 2019, 30, 2493–2505. [Google Scholar] [CrossRef]
- Reis, L.A.; Chiu, L.L.Y.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in Myocardial Tissue Engineering. J. Tissue Eng. Regen. Med. 2016, 10, 11–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yerneni, S.S.; Whiteside, T.L.; Weiss, L.E.; Campbell, P.G. Bioprinting Exosome-like Extracellular Vesicle Microenvironments. Bioprinting 2019, 13, e00041. [Google Scholar] [CrossRef]
- Born, L.J.; McLoughlin, S.T.; Dutta, D.; Mahadik, B.; Jia, X.; Fisher, J.P.; Jay, S.M. Sustained Released of Bioactive Mesenchymal Stromal Cell-derived Extracellular Vesicles from 3D-printed Gelatin Methacrylate Hydrogels. J. Biomed. Mater. Res. Part A 2022, 110, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.A.; Kipke, D.R.; Brandon, T. Calcium Alginate Gel: A Biocompatible and Mechanically Stable Polymer for Endovascular Embolization. J. Biomed. Mater. Res. 2001, 54, 76–86. [Google Scholar] [CrossRef]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C. Incorporation of Small Extracellular Vesicles in Sodium Alginate Hydrogel as a Novel Therapeutic Strategy for Myocardial Infarction. Theranostics 2019, 9, 7403. [Google Scholar] [CrossRef]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K. Cardiac Recovery via Extended Cell-Free Delivery of Extracellular Vesicles Secreted by Cardiomyocytes Derived from Induced Pluripotent Stem Cells. Nat. Biomed. Eng. 2018, 2, 293. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.-H.; Rubert, M.; Müller, R. Optimization of Mechanical Stiffness and Cell Density of 3D Bioprinted Cell-Laden Scaffolds Improves Extracellular Matrix Mineralization and Cellular Organization for Bone Tissue Engineering. Acta Biomater. 2020, 114, 307–322. [Google Scholar] [CrossRef]
- Shapira, A.; Noor, N.; Asulin, M.; Dvir, T. Stabilization Strategies in Extrusion-Based 3D Bioprinting for Tissue Engineering. Appl. Phys. Rev. 2018, 5, 41112. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, S.J.; Cheng, H.-J.; Yoo, J.J.; Atala, A. 3D Bioprinted Functional and Contractile Cardiac Tissue Constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Boetker, J.P.; Colombo, S.; Harmankaya, N.; Rantanen, J.; Bohr, A. Anti-Tuberculosis Drug Combination for Controlled Oral Delivery Using 3D Printed Compartmental Dosage Forms: From Drug Product Design to in Vivo Testing. J. Control. Release 2017, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of Drug-Loaded Hydrogels with Stereolithographic 3D Printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Linero, I.; Chaparro, O. Paracrine Effect of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Bone Regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [Green Version]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine Mechanisms of Stem Cell Reparative and Regenerative Actions in the Heart. J. Mol. Cell. Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Anthony, D.F.; Shiels, P.G. Exploiting Paracrine Mechanisms of Tissue Regeneration to Repair Damaged Organs. Transplant. Res. 2013, 2, 10. [Google Scholar] [CrossRef]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A. Sustained Release of Endothelial Progenitor Cell-Derived Extracellular Vesicles from Shear-Thinning Hydrogels Improves Angiogenesis and Promotes Function after Myocardial Infarction. Cardiovasc. Res. 2018, 114, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Elkhoury, K.; Chen, M.; Koçak, P.; Enciso-Martínez, E.; Bassous, N.J.; Lee, M.C.; Byambaa, B.; Rezaei, Z.; Li, Y.; López, M.E.U. Hybrid Extracellular Vesicles-Liposome Incorporated Advanced Bioink to Deliver MicroRNA. Biofabrication 2022, 14, 45008. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Barroso, I.A.; Brunet, M.Y.; Peacock, B.; Federici, A.S.; Hoey, D.A.; Cox, S.C. Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a Gelma/Nanoclay Composite Hydrogel to Promote Bone Repair. Int. J. Mol. Sci. 2022, 23, 832. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Shao, L.; Zhang, Y.; Zhou, J.; Liu, B.; Pan, X.; Geng, Y.; Yu, X.; Li, Y. Exosomes Derived from Embryonic Stem Cells as Potential Treatment for Cardiovascular Diseases BT. In Exosomes in Cardiovascular Diseases: Biomarkers, Pathological and Therapeutic Effects; Xiao, J., Cretoiu, S., Eds.; Springer: Singapore, 2017; pp. 187–206. ISBN 978-981-10-4397-0. [Google Scholar]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal Stem Cell Exosome: A Novel Stem Cell-Based Therapy for Cardiovascular Disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.; Huang, C.; Guo, Y.; Rao, L. Engineered Extracellular Vesicles and Their Mimics in Cardiovascular Diseases. J. Control. Release 2022, 347, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Song, Y.; Wang, Q.; Chen, J.; Gao, J.; Tan, H.; Li, S.; Wu, Y.; Yang, H.; Huang, H.; et al. Engineering Extracellular Vesicles with Platelet Membranes Fusion Enhanced Targeted Therapeutic Angiogenesis in a Mouse Model of Myocardial Ischemia Reperfusion. Theranostics 2021, 11, 3916–3931. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes Derived from Alcohol-Treated Hepatocytes Horizontally Transfer Liver Specific MiRNA-122 and Sensitize Monocytes to LPS. Sci. Rep. 2015, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.; Momen-Heravi, F.; Kodys, K.; Szabo, G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-Exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J. Biol. Chem. 2016, 291, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Bondalapati, S.; Ruvinov, E.; Kryukov, O.; Cohen, S.; Brik, A. Rapid End-Group Modification of Polysaccharides for Biomaterial Applications in Regenerative Medicine. Macromol. Rapid Commun. 2014, 35, 1754–1762. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of Bioink Properties on Printability and Cell Viability for 3D Bioplotting of Embryonic Stem Cells. Biofabrication 2016, 8, 35020. [Google Scholar] [CrossRef]








| Bioink | Alg-RGD | Calcium Gluconate | Gelatin Type B | Matrigel™ |
|---|---|---|---|---|
| 1 | 1.7 | 0.36 | 0 | 0 |
| 2 | 1.5 | 0.3 | 3 | 0 |
| 3 | 1.5 | 0.3 | 1.5 | 10 |
| Bioink Solution # | Printing Parameters | ||
|---|---|---|---|
| Temperature (°C) | Pressure (bar) | Printing Velocity (mm/s) | |
| 1 | 20–22 | 0.6 | 20–22 |
| 2 | 28 | 0.5 | 12 |
| 3 | 26 | 0.5 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar, A.; Kryukov, O.; Cohen, S. Three-Dimensional Bio-Printed Cardiac Patch for Sustained Delivery of Extracellular Vesicles from the Interface. Gels 2022, 8, 769. https://doi.org/10.3390/gels8120769
Bar A, Kryukov O, Cohen S. Three-Dimensional Bio-Printed Cardiac Patch for Sustained Delivery of Extracellular Vesicles from the Interface. Gels. 2022; 8(12):769. https://doi.org/10.3390/gels8120769
Chicago/Turabian StyleBar, Assaf, Olga Kryukov, and Smadar Cohen. 2022. "Three-Dimensional Bio-Printed Cardiac Patch for Sustained Delivery of Extracellular Vesicles from the Interface" Gels 8, no. 12: 769. https://doi.org/10.3390/gels8120769
APA StyleBar, A., Kryukov, O., & Cohen, S. (2022). Three-Dimensional Bio-Printed Cardiac Patch for Sustained Delivery of Extracellular Vesicles from the Interface. Gels, 8(12), 769. https://doi.org/10.3390/gels8120769