The Basic Study of Liposome in Temperature-Sensitive Gel at Body Temperature for Treatment of Peritoneal Dissemination
Abstract
:1. Introduction
2. Results
2.1. Characterization of Liposome
2.2. Cytotoxicity of Liposomal Paclitaxel
2.3. Viscosity Changes in Temperature-Sensitive Gels
2.4. Paclitaxel Leak Behavior from Temperature-Sensitive Gels
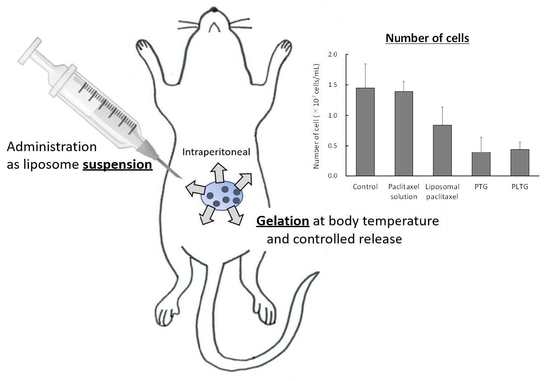
2.5. Antitumor Effect on Peritoneal Dissemination Model Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation and Characterization of Paclitaxel-Loaded Liposomes
4.4. Cytotoxicity
4.5. Preparation of Temperature-Sensitive Gel
4.6. The Viscosity of Temperature-Sensitive Gels
4.7. Paclitaxel Leak from PTG and PLTG
4.8. Peritoneal Dissemination Treatment Study (In Vivo)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, D.S.P.; Agarwal, R.; Kaye, S.B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006, 11, 925–934. [Google Scholar] [CrossRef]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survaival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Norman, A.R.; Cunningham, D.; Waters, J.S.; Oates, J.; Ross, P.J. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—Pooled analysis from three multicancer, randomized, controlled trials using individual patient data. J. Clin. Oncol. 2004, 22, 2395–2403. [Google Scholar] [CrossRef]
- Burges, A.; Schmalfeldt, B. Ovarian cancer: Diagnosis and treatment. Dtsch. Arztebl. Int. 2011, 108, 635–641. [Google Scholar]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Yonemura, Y.; Endo, Y.; Obata, T.; Sasaki, T. Recent advances in the treatment of peritoneal dissemination of gastrointestinal cancers by nucleoside antimetabolites. Cancer Sci. 2007, 98, 11–18. [Google Scholar] [CrossRef]
- Zieker, D.; Königsrainer, I.; Tritschler, I.; Löffler, M.; Beckert, S.; Traub, F.; Nieselt, K.; Bühler, S.; Weller, M.; Gaedcke, J.; et al. Phophpglycerate kinase 1 a promoting enzyme for peritoneal dissemination in gastric cancer. Int. J. Cancer 2010, 126, 1513–1520. [Google Scholar]
- Kurashige, J.; Mima, K.; Sawada, G.; Takahashi, Y.; Eguchi, H.; Sugimachi, K.; Mori, M.; Yanagihara, K.; Yashiro, M.; Hirakawa, K.; et al. Epigenetic modulation and repression of miR-200b by cancer-associated fibroblasts contribute to cancer invasion and peritoneal dissemination in gastric cancer. Carcinogenesis 2015, 36, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.; Kim, C.; Kim, J.H.; Kwon, W.S.; Lee, W.S.; Kim, J.M.; Park, J.Y.; Kim, H.S.; Park, K.H.; Kim, T.S.; et al. Genetic alterations and their clinical implications in gastric cancer peritoneal carcinomatosis revealed by whole-exome sequencing of malignant ascites. Oncotarget. 2016, 7, 8055–8066. [Google Scholar] [CrossRef] [Green Version]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-years follow-up results of the randomized nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Kanda, M.; Kodera, Y.; Sakamoto, J. Updated evidence on adjuvant treatments for gastric cancer. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1549–1560. [Google Scholar] [CrossRef]
- Paoletti, X.; Oba, K.; Burzykowski, T.; Michiels, S.; Ohashi, Y.; Pignon, J.P.; Rougier, P.; Sakamoto, J.; Sargent, D.; Sasako, M.; et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA 2010, 303, 1729–1737. [Google Scholar] [PubMed]
- Hartgrink, H.H.; Jamsem, E.P.; van Grieken, N.C.; van de Velde, C.J. Gastric cancer. Lancet 2009, 374, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Kanda, M.; Mizuno, A.; Fujii, T.; Shimoyama, Y.; Yamada, S.; Tanaka, C.; Kobayashi, D.; Koike, M.; Iwata, N.; Niwa, Y.; et al. Tumor infiltrative pattern predicts sites of recurrence after curative gastrectomy for stage 2 and 3 gastric cancer. Ann. Surg. Oncol. 2016, 23, 1937–1940. [Google Scholar] [CrossRef]
- Washwa, R.; Song, S.; Lee, J.S.; Yao, Y.; Wei, Q.; Ajani, J.A. Gastric cancer-molecular and clinical dimensions. Nat. Rev. Clin. Oncol. 2013, 10, 643–655. [Google Scholar]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Jaaback, K.; Johnson, N.; Lawrie, T.A. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 11, CD005340. [Google Scholar]
- Trimble, E.L.; Alvarez, R.D. Intraperitoneal chemotherapy and the NCI clinical announcement. Gynecol. Oncol. 2006, 103, S18–S19. [Google Scholar] [CrossRef]
- Rossi, L.; Verrico, M.; Zaccarelli, E.; Papa, A.; Colonna, M.; Strudel, M.; Vici, P.; Bianco, V.; Tomao, F. Bevacizumab in ovarian cancer: A critical review of phase III studies. Oncotarget 2017, 8, 12389–12405. [Google Scholar] [CrossRef] [Green Version]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, E.; Hong, D.; Le, H.; Chisholm, G.; Iyer, R.; Naing, A.; Hwu, P.; Jazaeri, A. Characteristics and outcomes of patients with recurrent ovarian cancer undergoing early phase immune checkpoint inhibitor clinical trials. Gynecol. Oncol. 2018, 151, 407–413. [Google Scholar] [CrossRef]
- Lambert, L.A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J. Clin. 2015, 65, 284–298. [Google Scholar] [CrossRef] [PubMed]
- De Smet, L.; Ceelen, W.; Remon, J.P.; Vervaet, C. Optimization of drug delivery systems for intraperitoneal therapy to extend the residence time of the chemotherapeutic agent. Sci. World J. 2013, 2013, 720858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, K.; Hunt, C.A.; Strubbe, A.; MacGregor, R.D. Lymphatic transport of liposome-encapsulated drugs following intraperitoneal administration-effect of lipid composition. Pharm. Res. 1985, 2, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sadzuka, Y.; Nakai, S.; Miyagishima, A.; Nozawa, Y.; Hirota, S. Effects of administered route on tissue distribution and antitumor activity of polyethyleneglycol-coated liposomes containing adriamycin. Cancer Lett. 1997, 111, 77–86. [Google Scholar] [CrossRef]
- Rezaeian, M.; Sedaghatkish, A.; Soltani, M. Numerical modeling of high intensity focused ultrasound mediated intraperitoneal delivery of thermosensitive liposomal doxorubicin for cancer chemotherapy. Drug Deliv. 2019, 26, 898–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermos-sensitive hydrogels for drug delivery. J. Mater. Chem. B. 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Wlstad, N.L.; Fowers, K.D. OncoGel (ReGel/paclitaxel)-Clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009, 61, 785–794. [Google Scholar] [CrossRef]
- Vukelja, S.J.; Anthony, S.P.; Arseneau, J.C.; Berman, B.S.; Cunningham, C.C.; Nemunaitis, J.J.; Fowers, K.D. Phase 1 study of escalating-dose OncoGel®(ReGel®/paclitaxel) depot injection, a controlled-release formulation of paclitaxel, for local management of superficial solid tumor lesions. Anti-Cancer Drugs 2007, 18, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Kageyama, S.; Suzuki, H. Preparation and physicochemical properties of a reversible, thermo-setting, in situ gelling ophthalmic solution containing timolol maleate. Mater. Technol. 1999, 17, 445–452. [Google Scholar]
- Chambers, S.K.; Chow, H.-H.S.; Janicek, M.F.; Cragun, J.M.; Hatch, K.D.; Cui, H.; Laughren, C.; Clouser, M.C.; Cohen, J.L.; Wright, H.M. Phase I trial of intraperitoneal pemetrexed, cisplatin, and paclitaxel in optimally debulked ovarian cancer. Clin. Cancer Res. 2012, 18, 2668–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, I.; Sadzuka, Y. Enhanced antitumor activity of different double arms polyethyleneglycol-modified liposomal doxorubicin. Int. J. Pharm. 2013, 441, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Miller, G.L. Protein determination for large numbers of samples. Anal. Chem. 1959, 31, 964. [Google Scholar] [CrossRef]






| Methylcellulose 15 (%(w/w)) | Methylcellulose 400 (%(w/w)) | Sodium Citrate (%(w/w)) | Macrogol 4000 (%(w/w)) | |
|---|---|---|---|---|
| A | 0.35 | 0.35 | 1.75 | 1.00 |
| B | 0.70 | 0.70 | 3.50 | 2.00 |
| C | 0.525 | 0.525 | 8.75 | 1.50 |
| D | 0.70 | 0.70 | 5.00 | 2.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiyama, I.; Ando, K.; Sadzuka, Y. The Basic Study of Liposome in Temperature-Sensitive Gel at Body Temperature for Treatment of Peritoneal Dissemination. Gels 2022, 8, 252. https://doi.org/10.3390/gels8050252
Sugiyama I, Ando K, Sadzuka Y. The Basic Study of Liposome in Temperature-Sensitive Gel at Body Temperature for Treatment of Peritoneal Dissemination. Gels. 2022; 8(5):252. https://doi.org/10.3390/gels8050252
Chicago/Turabian StyleSugiyama, Ikumi, Kaana Ando, and Yasuyuki Sadzuka. 2022. "The Basic Study of Liposome in Temperature-Sensitive Gel at Body Temperature for Treatment of Peritoneal Dissemination" Gels 8, no. 5: 252. https://doi.org/10.3390/gels8050252
APA StyleSugiyama, I., Ando, K., & Sadzuka, Y. (2022). The Basic Study of Liposome in Temperature-Sensitive Gel at Body Temperature for Treatment of Peritoneal Dissemination. Gels, 8(5), 252. https://doi.org/10.3390/gels8050252