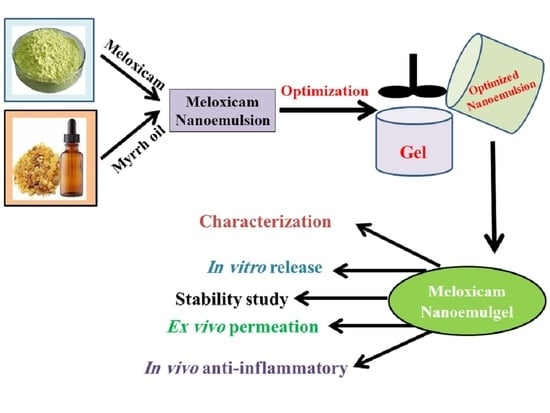
1. Introduction
Conventional dosage forms intended for transdermal and topical application were greatly applied formerly, including ointments, creams and patches; however, certain weaknesses have limited their administration. Among these problems are poor solubility, bad penetration and an inadequate drug-loading capacity, in addition to certain stability problems and poor spreadability, which necessitate overcoming these drawbacks to reach more comfortable and reproducible activity [
1].
Nanotechnology is an advanced approach, intended for design, and it develops matters in a nanoscale range, acting to address the undesired properties of active constituents and maximize their therapeutic effects [
2]. Several nanocarriers can be applied for these purposes, namely liposome, ethosome, niosome, nanoparticles and a nanoemulsion [
3].
A nanoemulsion (NE) is one of the lately developed nanocarriers that have gained a lot of attention owing to various merits, mostly their small particle size, which would provide better absorption and, consequently, improve the bioavailability of the incorporated drug [
4]. Moreover, it could improve drug solubility, offer controlled release of drug and provide protection against degradation [
5]. NE is a thermodynamically stable system formed of an aqueous phase, oily phase, surfactant and sometimes co-surfactant to form a single-phase system [
6]. It exhibits more stability over conventional emulsions in terms of flocculation, phase separation, sedimentation and creaming [
7]. It is a promising drug delivery system, which could be administered via different routes of administration, including parenteral [
8], oral [
9] and transdermal routes [
10]. However, for a transdermal delivery of the drug, it is practically better to incorporate the developed nanoemulsion into a hydrogel base to form a novel dosage form, termed a nanoemulgel (NEG).
NEG is a contemporary formulation anticipated for treating skin disorders. It can enhance permeability of the drug through the skin [
11] in addition to its good rheological properties and the dual effects of both the nanoemulsion and hydrogel [
12]. It can provide better drug adhesion to the skin, which leads to a higher concentration gradient towards the skin. Additionally, integrating a drug into an NEG formulation could improve its stability and ensure a controlled release [
13]. Nowadays, NEGs have been involved in the treatment of several infections incorporating a variety of agents, such as antibacterial, antifungal, anticancer and anti-inflammatory agents [
14].
Presently, the treatment of several diseases has been focused on exploring natural products, owing to their safety and evidenced pharmacological activities [
15]. Eucalyptus is a natural medicinal plant that belongs to the Myrtaceae family, renowned for its essential oils that have been extracted from
Eucalyptus globulus and have shown great potential in the field of nanotechnology [
16]. Eucalyptus essential oil (EEO) has proven to be effective in treating certain skin disorders, such as dermatitis [
17]. In addition, it has exhibited antioxidant, antibacterial and antifungal behavior [
18]. Its main constituent is known as Eucalyptol (1,8-cineole), which has been proven to show analgesic and anti-inflammatory influences, since it inhibits the synthesis of certain cytokines in inflammatory cells [
19,
20]. EEO incorporated into various nanocarriers, such as a microemulsion, nanoemulsion and nanoemulgel, has confirmed its improved performance and, consequently, augmentation of the therapeutic influence [
21,
22].
Meloxicam (MX) is regarded as one of the potent non-steroidal anti-inflammatory drugs (NSAIDs) related to the enolic acid group and proposed for treating various inflammatory disorders [
23]. It exerts a potent inhibition of cyclo-oxygenaze-2 more than cyclo-oxygenaze-1, which results in an identical effectiveness as other NSAIDs, however, with lower toxicity [
24]. Owing to the recurrent gastrointestinal complications that have been associated with the oral administration of MX, other alternative routes of administration seem to be necessary. Therefore, delivering MX through the skin via incorporation into a transdermal formulation has been viewed as one of the supportive ways to evade the disadvantages of oral administration, gain the benefits of applying the drug directly to the target site and lessen the dosing frequency, which would improve the compliance of the patient [
25,
26]. There have not been several studies that have investigated the influence of MX when incorporated into a nanoemulgel formulation; however, Drais et al. used almond and peppermint oil for preparing an MX-NEG [
27]. As far as we know, no previous literature has investigated the synergistic effect between MX and eucalyptus essential oil for boosting the anti-inflammatory effect.
For a more proficient study, a specific trend could be employed in order to design, optimize and examine the interrelation between certain factors and their related responses, which is well-known as quality by design (QbD) [
28]. Central Composite Design (CCD) is one of these tools that help in such optimization, depending on specific mathematical equations and statistical analyses for inspecting the model [
29].
In these contexts, MX loaded into various EEO-based NEs was developed through employing a 22 full factorial design and investigating the influence of specific variables on the NE characterization. At that point, one optimized NE formulation was selected and integrated with the hydrogel base and went through definite physical and chemical evaluations. To conclude, the anti-inflammatory activity of the developed MX-NEG containing EEO was investigated to validate its potential as an efficient anti-inflammatory agent.
4. Materials and Methods
4.1. Materials
Meloxicam was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Eucalyptus essential oil was obtained from NOW® Essential Oils (NOW Foods, Bloomingdale, IL, USA). Diethylene Glycol Monoethyl Ether (Transcutol® P) was procured from Gattefosse SAS (Saint-priest Cedex, Lyon, France). Polysorbate 80 (Tween 80), Hydroxpropyl methylcellulose (HPMC) (K15M) and poly ethylene glycol 400 (PEG 400) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals were of the finest grade available.
4.2. Solubility Examination of MX in the Formulation Constituents
The solubility of MX was assessed in EEO, tween 80, PEG 400 and transcutol
® P. An excess amount of MX was shaken with 1 mL of each component separately for 48 h at 25 °C, using a shaker water bath (Gesellschaft fur Labortechnik mbH, Burgwedel, Germany). Next, samples were centrifuged for 15 min at 2000 rpm using a centrifuge (Andreas Hettich GmbH, Co.KG, Tuttlingen, Germany); then, they were filtered and diluted with methanol. The samples were analyzed spectrophotometrically (U.V. Spectrophotometer, JENWAY 6305, Bibby Scientific Ltd., Stone, Staffs, UK) at λ
max of 360 nm [
30].
4.3. Experimental Design
A two-factor, two-level (2
2) factorial design was implemented in order to optimize the developed NE formulations using Response surface methodology (RSM), which was mainly CCD. Two factors representing the independent variables were selected (oil concentration, A, and surfactant concentration, B), which were examined at two levels, low (−1) and high (+1), as shown in
Table 8. The influence of these factors on the response of the prepared NE was investigated, such as particle size (Y
1) and in vitro drug release (Y
2). The Design-Expert version 12.0 software (Stat-Ease, Minneapolis, MN, USA) was employed for carrying out the current optimization via generating statistical analyses of the data using an Analysis of variance (ANOVA) test. Subsequently, modeling graphs were constructed in addition to mathematical equations that provide an illustration for the response as follows:
where Y denotes the selected response, and b
0 denotes the intercept; b
1, b
2, b
12, b
11 and b
22 are the regression coefficients. A and B characterize the studied factors; AB symbolize the interactions between the main factors, while A
2 and B
2 indicate the polynomial terms.
4.4. Development of an EEO-Based NE Loaded with MX
Various NE preparations incorporating MX were developed using the specified amounts of constituents. A total of 1% (
w/
w) of MX was added into the specified amount of EEO and transcutol
® P (0.5 g), which is an excellent solvent, and was well-mixed to form the oily phase. Different amounts of tween 80 acting as a surfactant and PEG 400 (0.5 g) as a co-surfactant were added to distilled water, followed by vortexing to form the aqueous phase. Both phases were mixed together, and the volume was adjusted to reach 10 g with distilled water. Mixing of the two phases continued for 15 min using a high shear homogenizer (T 25 digital Ultra-Turrax, IKA, Staufen, Germany) at 20,000 rpm. Promptly, the NE was formed next to homogenization; afterward, it was subjected to 1 min of sonication using a probe sonicator (XL-2000, Qsonica, Newtown, CT, USA) [
47]. The matrix of 11 experimental formulations was constructed using CCD along with the values of their observed responses, as clarified in
Table 1.
4.5. NE Characterization
Particle size and Polydispersity Index (PDI) Determination
A Zetasizer apparatus (Malvern Instruments Ltd., Worcestershire, UK) was operated in order to analyze the particle size and PDI of all of the generated MX-NE formulations. These characters were measured using dynamic light scattering, which was adjusted at 25 °C, using a scattering angle of 90° [
48].
4.6. In Vitro Release
An Agilent Fiber optics dissolution system (Agilent Technologies, San Francisco, CA, USA) was run to determine the percentage of MX released from all of the generated NE formulations. Glass tubes were used in the system as substitutes to the baskets. The tubes were sealed from one side with a cellophane membrane (MWCO 2000–15,000), to which 2 mL of the preparation was added. A vehicle of 500 mL of phosphate buffer saline (PBS) of pH 7.4 was added into the system and kept at 37 ± 0.5 °C and then rotated at 50 rpm. At definite intervals of time (0.25, 0.5, 1, 2 and 6 h), the samples were examined at λ
max of 360 nm. The same procedure was performed to measure the percentage of MX released from the developed NEG formulation [
49].
4.7. Development of MX-NEG
MX-NEG was fabricated by incorporating a gelling agent into the optimized EEO-based NE loaded with MX. Approximately 4%
w/
w of HPMC, which behaves as a gelling agent, was steadily scattered into 10 mL of distilled water to provide an HPMC hydrogel and was then mixed with an EEO-based NE containing the drug. The mixture was well-mixed for 5 min using Heidolph RZR 1 (Heidolph Instruments, Schwabach, Germany) in order to obtained consistent NEG (20 g) [
50]. For a distinctive evaluation of the MX-NEG efficacy, a placebo NEG (without MX) was developed using same procedure of preparing MX-NEG and the same amount of constituents (EEO (1.5 g), Tween 80 (1.0 g), PEG 400 (0.5g) and transcutol
® P (0.5 g)). Moreover, the MX gel (MX-G) was fabricated by scattering MX over 2% HPMC gel.
4.8. Evaluating the Developed MX-NEG
4.8.1. Physical Examination
The prepared MX-NEG formulation was visually examined for its appearance, color and homogeneity.
4.8.2. pH Measurement
A standardized pH meter (MW802, Milwaukee Instruments, Szeged, Hungary) was utilized to determine the formulation’s pH and confirm its safety to be applied topically on the skin [
51].
4.8.3. Drug Content
A precise amount of the NEG preparation (1 g) was diluted in 100 mL of phosphate buffer saline (PBS) and then filtered using 0.45 micro-syringe filters. The drug content was assayed spectrophotometrically at an λmax of 360 nm [
52]. For the blank sample (sample without the drug), the identical technique was carried out, and then, the drug content was measured using the following equation: Drug content = (Actual/Theoretical) × 100.
4.8.4. Viscosity
In order to measure the viscosity of the developed MX-NEG formulation, a Brookfield viscometer (DV-II+ Pro, Middleboro, USA) was operated at 25 °C, using spindle R5, and allowed to rotate at 0.5 rpm [
49].
4.8.5. Spreadability Test
The spreadability of the formulation is an indicator of its capability to spread readily when applied on the skin by determining the spreading diameter. Concisely, a certain amount of the NEG formulation (1 g) was held in-between two glass slides (25 cm × 25 cm), and a certain load was fixed over the system for 1 min. The value of the spreadability was obtained by measuring the spreading diameter of the formulation over the affected area [
53].
4.9. In Vitro Release Study from Different Developed Formulations
The percentage of the drug released from the different prepared formulations was estimated via an Agilent Fiber optics dissolution system (Agilent Technologies, San Francisco, CA, USA). Definite amounts of the optimized EEO-based NE, MX-G and MX-NEG formulations were examined, whereby the same technique mentioned previously in
Section 2.5 was followed.
4.10. Stability Study of MX-NEG
The developed MX-NEG was verified for its stability with regards to the evaluated characterization parameters, including physical examination, pH, drug content, viscosity, and spreadability, in addition to the in vitro release study. These factors were measured after being stored for 1 and 3 months at two different conditions: 4 ± 1 °C and at 25 ± 1 °C. This exploration was performed in accordance with the guidelines of the International Conference on Harmonization (ICH) [
54].
4.11. Ex Vivo Study
4.11.1. Preparation of Rat Skin
In the present study, the skin of Male Wister rats was utilized. First, an electric clipper was used to carefully remove the animal’s dorsal hair. Then, the rats were sacrificed, and the skin was cut out. The skin was hydrated overnight in a phosphate buffer (pH 7.4) at 4 °C after removing the adipose tissue [
50].
4.11.2. Permeation Study
Adapted Franz diffusion cells established in our lab were employed to detect the permeation of MX from the gel and NEG through the skin of male Wister rats, as illustrated in
Figure 10 [
55,
56,
57]. The diffusion system was kept at 37 ± 0.5 °C and contained 100 mL of phosphate buffer (pH 7.4) with 0.02% sodium azide. Glass tubes were suspended in the media in the apparatus. The rat skin was attached to the diffusion cell, wherein the upside of the skin faced the formulation, while its dermis was in front of the receptor media. The examined formulation was enclosed with the rat skin membrane and fixed to the glass tubes. In order to evade evaporation of water, the cells were covered with Parafilm (Bemis, Oshkosh, WI, USA), and the system was stirred at 100 rpm. [
58]. The steady state transdermal flux (SSTF) and enhancement ratio (ER) are two parameters relating to the ex vivo permeation study that were evaluated, since SSTF represents the amount of permeated drug/(area × time); whereas ER denotes SSTF from the test/SSTF from the control.
4.12. Animal
Aiming to carry out animal experiments, 220–250 g Male Wister rats were supplied from an animal breeding center, from the College of Science, King Faisal University. Animals were maintained under a controlled housing condition, in which the light and dark cycle was adjusted to be 12:12 h, and at an ambient temperature (25 ± 2 °C). Regarding the ethical statement, all experiments were conducted in accordance with the guidelines and were ethically approved by the Research Ethics Committee (REC) of King Faisal University, approval number KFU-REC-2021-DEC-EA000308.
4.13. In Vivo Study
4.13.1. In Vivo Skin Irritation Test
It is very important to guarantee the safety of the formulation, especially if intended for topical application. This study was conducted using male Wister rats by preparing them one day earlier. Then, the dorsal hair of the animals was shaved using an electric clipper, and the examined formulation (MX-NEG) was evenly spread over the shaved area. The animals were observed for 7 days after being treated topically with the formulation in order to detect any responses relating to irritation erythema (redness) or edema. Any perceived response was demonstrated based on a scale that varied from 0 to 3, which signified that the sensitivity reactions could be no, a minor, a moderate or a severe erythema reaction, with or without edema [
6].
4.13.2. In Vivo Anti-Inflammatory Study: Carrageenan-Induced Rat Hind Paw Edema Method
The EEO and MX anti-inflammatory activity was evaluated by performing the carrageenan-induced rat hind-paw edema model using male Wister rats. This method was conducted based on former reports adopted from Shehata et al. [
59], wherein an edema was induced in rat hind-paws half an hour prior to drug administration by means of injecting 0.5%
w/
v carrageenan in saline subcutaneously into the left hind paw [
60]. Rats were divided randomly into four groups, where n = 6, as follows:
Group I was the untreated group (control group).
Group II was treated with MX-G (5.6 mg/kg).
Group III was the placebo group, which was treated with NEG free from the drug.
Group IV was treated with MX-NEG (5.6 mg/kg) [
61].
At varied time intervals (0, 1, 2, 3, 4, 6 and 12 h), the inflammatory reaction was evaluated. This was accomplished using a digital caliber to detect the variations in the volume of the rat paw next to the transdermal application. The following equation was utilized to validate the % of inflammation:
where Vt denotes the volume of the carrageenan-treated hind paw, whereas V0 characterizes the hind paw at time zero.
4.14. Statistics
All experiments were confirmed by performing at least three independent trials as the mean ± SD. A student’s t-test was employed to detect the statistical differences between the groups. A one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) as a post-hoc test, was applied to compare data from the treated and control groups. These evaluations were established using SPSS statistics software, version 9 (IBM Corporation, Armonk, NY, USA). A statistically significant difference between groups was approved if p < 0.05.