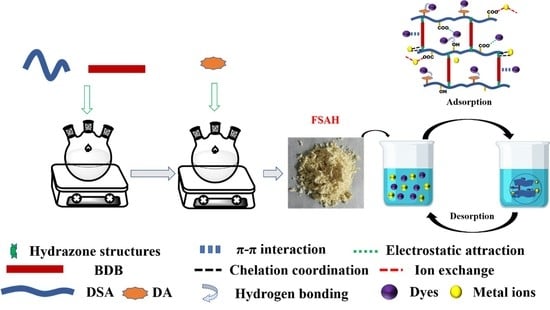
Highly Efficient Adsorption of Heavy Metals and Cationic Dyes by Smart Functionalized Sodium Alginate Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations
2.1.1. SEM and EDS–Mapping Analysis
2.1.2. FT-IR and XPS Analysis
2.1.3. TG and BET Analysis
2.2. Adsorption Performance Study
2.2.1. DA Mass Ratio
2.2.2. pH
2.2.3. Adsorption Isotherm
2.2.4. Adsorption Kinetic
2.2.5. Thermodynamic Adsorption
2.2.6. Reuse Adsorption
2.3. Adsorption Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of DSA
4.3. Preparation of FSAH
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, R.; Jiang, W.; Wang, L.; Peng, J.; Chen, Y. Effect of pyrolusite loading on sewage sludge-based activated carbon in Cu(II), Pb(II), and Cd(II) adsorption. Environ. Prog. Sustain. Energy 2013, 32, 1066–1073. [Google Scholar] [CrossRef]
- Shahzad, A.; Miran, W.; Rasool, K.; Nawaz, M.; Jang, J.; Lim, S.-R.; Lee, D.S. Heavy metals removal by EDTA-functionalized chitosan graphene oxide nanocomposites. RSC Adv. 2017, 7, 9764–9771. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, B.; Jarvis, K.; Majewski, P. Plasma polymer-functionalized silica particles for heavy metals removal. ACS Appl. Mater. Interfaces 2015, 7, 4265–4274. [Google Scholar] [CrossRef] [PubMed]
- Singha, N.R.; Karmakar, M.; Mahapatra, M.; Mondal, H.; Dutta, A.; Deb, M.; Mitra, M.; Roy, C.; Chattopadhyay, P.K. An in situ approach for the synthesis of a gum ghatti-g-interpenetrating terpolymer network hydrogel for the high-performance adsorption mechanism evaluation of Cd(ii), Pb(ii), Bi(iii) and Sb(iii). J. Mater. Chem. A 2018, 6, 8078–8100. [Google Scholar] [CrossRef]
- Singha, N.R.; Chattopadhyay, P.K.; Dutta, A.; Mahapatra, M.; Deb, M. Review on additives-based structure-property alterations in dyeing of collagenic matrices. J. Mol. Liq. 2019, 293, 111470. [Google Scholar] [CrossRef]
- Shi, P.; Hu, X.; Duan, M. A UIO-66/tannic acid/chitosan/polyethersulfone hybrid membrane-like adsorbent for the dynamic removal of dye and Cr (VI) from water. J. Clean. Prod. 2021, 290, 125794. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Saya, L.; Malik, V.; Singh, A.; Singh, S.; Gambhir, G.; Singh, W.R.; Chandra, R.; Hooda, S. Guar gum based nanocomposites: Role in water purification through efficient removal of dyes and metal ions. Carbohydr. Polym. 2021, 261, 117851. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard. Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, A.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Simultaneous adsorption of heavy metals and organic dyes by beta-Cyclodextrin-Chitosan based cross-linked adsorbent. Carbohydr. Polym. 2021, 255, 117486. [Google Scholar] [CrossRef]
- Chen, M.; Bi, R.; Zhang, R.; Yang, F.; Chen, F. Tunable surface charge and hydrophilicity of sodium polyacrylate intercalated layered double hydroxide for efficient removal of dyes and heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126384. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Mason, T.J.; Calinescu, I.; Lavric, V.; Vinatoru, M.; Melinescu, A.; Culita, D.C. Ultrasound assisted preparation of calcium alginate beads to improve absorption of Pb(+2) from water. Ultrason. Sonochem. 2020, 68, 105191. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.; Yang, H.; Luo, X.; Lin, X. Highly efficient extraction of thorium from aqueous solution by fungal mycelium-based microspheres fabricated via immobilization. Chem. Eng. J. 2019, 368, 37–50. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent—A review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; Chu, Y.; Yue, Y.; Shi, W.; Zhou, J. Synthesis of Sulfonylhydrazone Type Probe with High Selectivity for Rapid Detection of Mercury and Its Application in Adsorption and HeLa Cell. Chin. J. Org. Chem. 2021, 41, 1138–1145. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; He, J. 1-[(2-Hydroxy-phenylimino)-methyl]-naphthalen-2-ol: Application in detection and adsorption of aluminum ions. Res. Chem. Intermed. 2021, 47, 4333–4347. [Google Scholar] [CrossRef]
- Wang, H.; Xue, S.; Zhou, X.; Liu, J.; Xie, Z. Synthesis of highly selective copper ion probe and its application in adsorption. Chin. J. Lumin. 2021, 42, 1427. [Google Scholar] [CrossRef]
- Ma, J.; Fang, S.; Shi, P.; Duan, M. Hydrazine-Functionalized guar-gum material capable of capturing heavy metal ions. Carbohydr. Polym. 2019, 223, 115137. [Google Scholar] [CrossRef]
- Wu, C.; Wang, H.; Wei, Z.; Li, C.; Luo, Z. Polydopamine-mediated surface functionalization of electrospun nanofibrous membranes: Preparation, characterization and their adsorption properties towards heavy metal ions. Appl. Surf. Sci. 2015, 346, 207–215. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Zhong, X.; Hu, J.; Shi, F.; Mi, H. Preparation and catalytic performance of alginate-based Schiff Base. Carbohydr. Polym. 2019, 208, 42–49. [Google Scholar] [CrossRef]
- Wilson, L.D.; Pratt, D.Y.; Kozinski, J.A. Preparation and sorption studies of beta-cyclodextrin-chitosan-glutaraldehyde terpolymers. J. Colloid Interface Sci. 2013, 393, 271–277. [Google Scholar] [CrossRef]
- Gorzalski, A.S.; Donley, C.; Coronell, O. Elemental composition of membrane foulant layers using EDS, XPS, and RBS. J. Membr. Sci. 2017, 522, 31–44. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Gou, S.; Zhou, L.; Tang, L.; Liu, T.; Liu, L.; Duan, M. Amidoxime-functionalized polyacrylamide-modified chitosan containing imidazoline groups for effective removal of Cu2+ and Ni2+. Carbohydr. Polym. 2021, 252, 117160. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Zhou, Y.; Lei, J.; Pu, S. Cyclodextrin modified filter paper for removal of cationic dyes/Cu ions from aqueous solutions. Water Sci. Technol. 2018, 78, 2553–2563. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, M.; Zhao, J.; Chen, J.; Zeng, G.; Huang, H.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci. 2018, 427, 535–544. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57U, 385–470. [Google Scholar] [CrossRef]
- Lin, L.; Tang, S.; Wang, X.; Sun, X.; Yu, A. Hexabromocyclododecane alters malachite green and lead(II) adsorption behaviors onto polystyrene microplastics: Interaction mechanism and competitive effect. Chemosphere 2021, 265, 129079. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Hu, L.; Gao, L.; Du, B.; Wei, Q. Mechanism of Pb(ii) and methylene blue adsorption onto magnetic carbonate hydroxyapatite/graphene oxide. RSC Adv. 2015, 5, 9759–9770. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.; Tavares, A.M.; Bruns, R.E. The removal of the indigo carmine dye from aqueous solutions using cross-linked chitosan: Evaluation of adsorption thermodynamics using a full factorial design. J. Hazard. Mater. 2008, 153, 566–574. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.; Li, R.; Xing, Y. Preparation and adsorption properties of citrate-crosslinked chitosan salt microspheres by microwave assisted method. Int. J. Biol. Macromol. 2020, 152, 1146–1156. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Zhu, Z.; Shi, W.; Liu, Y.; Liu, M.; Mo, X. Effective removal of metal ions and cationic dyes from aqueous solution using different hydrazine-dopamine modified sodium alginate. Int. J. Biol. Macromol. 2022, 195, 317–328. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, S.; Cheng, W.; Zhang, L.; Meng, P.; Zhang, T.; Yu, H.; Peng, D. Theoretical calculations, molecular dynamics simulations and experimental investigation of the adsorption of cadmium(ii) on amidoxime-chelating cellulose. J. Mater. Chem. A 2019, 7, 13714–13726. [Google Scholar] [CrossRef]
- Dai, J.; Yan, H.; Yang, H.; Cheng, R. Simple method for preparation of chitosan/poly(acrylic acid) blending hydrogel beads and adsorption of copper(II) from aqueous solutions. Chem. Eng. J. 2010, 165, 240–249. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, Y.; Zhai, T.; Shen, F.; Zeng, Y.; Fu, H.; Yao, J. Silver Nanoparticles Stabilized by Thermoresponsive Microgel Particles: Synthesis and Evidence of an Electron Donor-Acceptor Effect. Macromol. Rapid Commun. 2007, 28, 2339–2345. [Google Scholar] [CrossRef]
- Ling, C.; Liu, F.-Q.; Long, C.; Chen, T.-P.; Wu, Q.-Y.; Li, A.-M. Synergic removal and sequential recovery of acid black 1 and copper (II) with hyper-crosslinked resin and inside mechanisms. Chem. Eng. J. 2014, 236, 323–331. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing Functionalized Graphene as Electrodes for High-Performance Supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Bayatpour, S.; Qian, X.; Frigo-Vaz, B.; Wang, P. Activated carbon fibers via reductive carbonization of cellulosic biomass for adsorption of nonpolar volatile organic compounds. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125908. [Google Scholar] [CrossRef]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of Sodium Acrylate and Acrylamide Copolymer/GO Hydrogels and Their Effective Adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959. [Google Scholar] [CrossRef]
- Liu, M.; Xie, Z.; Ye, H.; Li, W.; Shi, W.; Liu, Y.; Zhang, Y. Waste polystyrene foam—Chitosan composite materials as high-efficient scavenger for the anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127155. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Pan, L.; Wang, H.; Bin, Y. MWCNTs reinforced conductive, self-healing polyvinyl alcohol/carboxymethyl chitosan/oxidized sodium alginate hydrogel as the strain sensor. J. Appl. Polym. Sci. 2020, 138, 49800. [Google Scholar] [CrossRef]
- Milani, S.A.; Karimi, M. Isotherm, kinetic and thermodynamic studies for Th(IV) sorption by amino group-functionalized titanosilicate from aqueous solutions. Korean J. Chem. Eng. 2017, 34, 1159–1169. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Zeng, X.; Kang, Y.; Wang, J.; Xie, H.; Zhang, Y. Efficient adsorption of methylene blue by xanthan gum derivative modified hydroxyapatite. Int. J. Biol. Macromol. 2020, 151, 1040–1048. [Google Scholar] [CrossRef]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Cheng, C.; Meng, Y.; Li, A. Preparation of new chelating fiber with waste PET as adsorbent for fast removal of Cu2+ and Ni2+ from water: Kinetic and equilibrium adsorption studies. Chem. Eng. J. 2012, 193–194, 31–38. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, M.; Carabineiro, S.A.C. Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/beta-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J. Hazard. Mater. 2021, 409, 124840. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Chen, Y.; Lan, T.; Duan, L.; Wang, F.; Zhao, B.; Zhang, S.; Wei, W. Adsorptive Removal and Adsorption Kinetics of Fluoroquinolone by Nano-Hydroxyapatite. PLoS ONE 2015, 10, e0145025. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Li, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Zhang, J.; Li, H.; Peng, Z.; Xu, R.; et al. Adsorption of Pb(II) by tourmaline-montmorillonite composite in aqueous phase. J. Colloid Interface Sci. 2020, 575, 367–376. [Google Scholar] [CrossRef]
- Hu, R.; Wang, X.; Dai, S.; Shao, D.; Hayat, T.; Alsaedi, A. Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions. Chem. Eng. J. 2015, 260, 469–477. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; ZA, A.L.; Alsuhybani, M.; Algamdi, M. Excellent adsorptive performance of a new nanocomposite for removal of toxic Pb(II) from aqueous environment: Adsorption mechanism and modeling analysis. J. Hazard. Mater. 2020, 389, 121896. [Google Scholar] [CrossRef]
- Thanarasu, A.; Periyasamy, K.; Manickam Periyaraman, P.; Devaraj, T.; Velayutham, K.; Subramanian, S. Comparative studies on adsorption of dye and heavy metal ions from effluents using eco-friendly adsorbent. Mater. Today Proc. 2021, 36, 775–781. [Google Scholar] [CrossRef]
- Sheikhi, A.; Safari, S.; Yang, H.; van de Ven, T.G.M. Copper Removal Using Electrosterically Stabilized Nanocrystalline Cellulose. ACS Appl. Mater. Interfaces 2015, 7, 11301–11308. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z. Preparation of composite aerogels based on sodium alginate, and its application in removal of Pb2+ and Cu2+ from water. Int. J. Biol. Macromol. 2018, 107, 741–747. [Google Scholar] [CrossRef]
- Ilgin, P.; Durak, H.; Gür, A. A Novel pH-Responsive p(AAm-co-METAC)/MMT Composite Hydrogel: Synthesis, Characterization and Its Absorption Performance on Heavy Metal İons. Polym.-Plast. Technol. Eng. 2015, 54, 603–615. [Google Scholar] [CrossRef]
- Ali, E.A.M.; Sayed, M.A.; Abdel-Rahman, T.M.A.; Hussein, R. Fungal remediation of Cd(ii) from wastewater using immobilization techniques. RSC Adv. 2021, 11, 4853–4863. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, F.; Yang, M.; Fang, P. POSS modified NixOy-decorated TiO2 nanosheets: Nanocomposites for adsorption and photocatalysis. Appl. Surf. Sci. 2021, 566, 150604. [Google Scholar] [CrossRef]
- Ji, Q.; Li, H. High surface area activated carbon derived from chitin for efficient adsorption of Crystal Violet. Diam. Relat. Mater. 2021, 118, 108516. [Google Scholar] [CrossRef]
- Abebe, M.W.; Kim, H. Methylcellulose/tannic acid complex particles coated on alginate hydrogel scaffold via Pickering for removal of methylene blue from aqueous and quinoline from non-aqueous media. Chemosphere 2021, 286, 131597. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Xie, Z.; Mo, X.; Feng, Y.; Peng, T.; Song, D. Highly Efficient Adsorption of Heavy Metals and Cationic Dyes by Smart Functionalized Sodium Alginate Hydrogels. Gels 2022, 8, 343. https://doi.org/10.3390/gels8060343
Shi T, Xie Z, Mo X, Feng Y, Peng T, Song D. Highly Efficient Adsorption of Heavy Metals and Cationic Dyes by Smart Functionalized Sodium Alginate Hydrogels. Gels. 2022; 8(6):343. https://doi.org/10.3390/gels8060343
Chicago/Turabian StyleShi, Tianzhu, Zhengfeng Xie, Xinliang Mo, Yulong Feng, Tao Peng, and Dandan Song. 2022. "Highly Efficient Adsorption of Heavy Metals and Cationic Dyes by Smart Functionalized Sodium Alginate Hydrogels" Gels 8, no. 6: 343. https://doi.org/10.3390/gels8060343
APA StyleShi, T., Xie, Z., Mo, X., Feng, Y., Peng, T., & Song, D. (2022). Highly Efficient Adsorption of Heavy Metals and Cationic Dyes by Smart Functionalized Sodium Alginate Hydrogels. Gels, 8(6), 343. https://doi.org/10.3390/gels8060343