Thermosensitive Shape-Memory Poly(stearyl acrylate-co-methoxy poly(ethylene glycol) acrylate) Hydrogels
Abstract
:1. Introduction
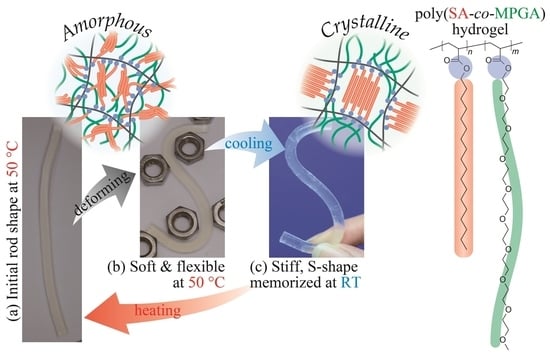
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Preparation of Poly(SA-co-MPGA) Gels
4.2. Swelling Properties in Water
4.3. Compression Test
4.4. DSC Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apsite, I.; Salehi, S.; Ionov, L. Materials for smart soft actuator systems. Chem. Rev. 2022, 122, 1349–1415. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.W.; Kim, H.C.; Zhai, L.; Ko, H.U.; Muthoka, R.M. Review of soft actuator materials. Int. J. Precis. Eng. Manuf. 2019, 20, 2221–2241. [Google Scholar] [CrossRef] [Green Version]
- Han, I.K.; Chung, T.; Han, J.; Kim, Y.S. Nanocomposite hydrogel actuators hybridized with various dimensional nanomaterials for stimuli responsiveness enhancement. Nano Converg. 2019, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Morimoto, N.; Jiang, L.; Kawahara, S.; Noritomi, T.; Yokoyama, H.; Mayumi, K.; Ito, K. Tough hydrogels with rapid self-reinforcement. Science 2021, 372, 1078–1081. [Google Scholar] [CrossRef]
- Nonoyama, T.; Gong, J.P. Tough double network hydrogel and its biomedical applications. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Fujiyabu, T.; Sakumichi, N.; Katashima, T.; Liu, C.; Mayumi, K.; Chung, U.I.; Sakai, T. Tri-branched gels: Rubbery materials with the lowest branching factor approach the ideal elastic limit. Sci. Adv. 2022, 8, eabk0010. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Tokuyama, H.; Mori, H.; Hamaguchi, R.; Kato, G. Prediction of the lower critical solution temperature of poly(N-isopropylacrylamide-co-methoxy triethyleneglycol acrylate) in aqueous salt solutions using support vector regression. Chem. Eng. Sci. 2021, 231, 116325. [Google Scholar] [CrossRef]
- Deng, K.; Rohn, M.; Gerlach, G. Design, simulation and characterization of hydrogel-based thermal actuators. Sens. Actuators B 2016, 236, 900–908. [Google Scholar] [CrossRef]
- Warren, H.; Shepherd, D.J.; in het Panhuis, M.; Officer, D.L.; Spinks, G.M. Porous PNIPAm hydrogels: Overcoming diffusion-governed hydrogel actuation. Sens. Actuators A 2020, 301, 111784. [Google Scholar] [CrossRef]
- Choi, J.G.; Spinks, G.M.; Kim, S.J. Mode shifting shape memory polymer and hydrogel composite fiber actuators for soft robots. Sens. Actuators A 2022, 342, 113619. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Liu, A.; He, S.; Shao, W. Ultrafast thermo-responsive bilayer hydrogel actuator assisted by hydrogel microspheres. Sens. Actuators B 2022, 357, 131434. [Google Scholar] [CrossRef]
- Tokuyama, H.; Sasaki, M.; Sakohara, S. Preparation of a novel composition-gradient thermosensitive gel. Colloids Surf. A 2006, 273, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Tokuyama, H.; Kato, Y. Preparation of thermosensitive polymeric organogels and their drug release behaviors. Eur. Polym. J. 2010, 46, 277–282. [Google Scholar] [CrossRef]
- Matsuda, A.; Sato, J.; Yasunaga, H.; Osada, Y. Order-disorder transition of a hydrogel containing an N-alkyl acrylate. Macromolecules 1994, 27, 7695–7698. [Google Scholar] [CrossRef]
- Osada, Y.; Matsuda, A. Shape memory in hydrogels. Nature 1995, 376, 219. [Google Scholar] [CrossRef]
- Kagami, Y.; Gong, J.P.; Osada, Y. Shape memory behaviors of crosslinked copolymers containing stearyl acrylate. Macromol. Rapid Commun. 1996, 17, 539–543. [Google Scholar] [CrossRef]
- Hasnat Kabir, M.; Gong, J.; Watanabe, Y.; Makino, M.; Furukawa, H. Hard-to-soft transition of transparent shape memory gels and the first observation of their critical temperature studied with scanning microscopic light scattering. Mater. Lett. 2013, 108, 239–242. [Google Scholar] [CrossRef]
- Hasnat Kabir, M.; Hazama, T.; Watanabe, Y.; Gong, J.; Murase, K.; Sunada, T.; Furukawa, H. Smart hydrogel with shape memory for biomedical applications. J. Taiwan Inst. Chem. Eng. 2014, 45, 3134–3138. [Google Scholar] [CrossRef]
- Shiblee, M.D.N.I.; Ahmed, K.; Yamazaki, Y.; Kawakami, M.; Furukawa, H. Light scattering and rheological studies of 3D/4D printable shape memory gels based on poly (N,N-dimethylacrylamide-co-stearyl acrylate and/or lauryl acrylates). Polymers 2021, 13, 128. [Google Scholar] [CrossRef]
- Kabir, M.H.; Ahmed, K.; Furukawa, H. The effect of cross-linker concentration on the physical properties of poly(dimethyl acrylamide-co-stearyl acrylate)-based shape memory hydrogels. Microelectron. Eng. 2016, 150, 43–46. [Google Scholar] [CrossRef]
- Lin, X.K.; Chen, L.; Zhao, Y.P.; Dong, Z.Z. Synthesis and characterization of thermoresponsive shape-memory poly(stearyl acrylate-co-acrylamide) hydrogels. J. Mater. Sci. 2010, 45, 2703–2707. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross–linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- James, H.M.; Guth, E. Theory of the elastic properties of rubber. J. Chem. Phys. 1943, 11, 455–481. [Google Scholar] [CrossRef]
- Tokuyama, H.; Nakahata, Y.; Ban, T. Diffusion coefficient of solute in heterogeneous and macroporous hydrogels and its correlation with the effective crosslinking density. J. Membr. Sci. 2020, 595, 117533. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokuyama, H.; Iriki, R.; Kubota, M. Thermosensitive Shape-Memory Poly(stearyl acrylate-co-methoxy poly(ethylene glycol) acrylate) Hydrogels. Gels 2023, 9, 54. https://doi.org/10.3390/gels9010054
Tokuyama H, Iriki R, Kubota M. Thermosensitive Shape-Memory Poly(stearyl acrylate-co-methoxy poly(ethylene glycol) acrylate) Hydrogels. Gels. 2023; 9(1):54. https://doi.org/10.3390/gels9010054
Chicago/Turabian StyleTokuyama, Hideaki, Ryo Iriki, and Makino Kubota. 2023. "Thermosensitive Shape-Memory Poly(stearyl acrylate-co-methoxy poly(ethylene glycol) acrylate) Hydrogels" Gels 9, no. 1: 54. https://doi.org/10.3390/gels9010054
APA StyleTokuyama, H., Iriki, R., & Kubota, M. (2023). Thermosensitive Shape-Memory Poly(stearyl acrylate-co-methoxy poly(ethylene glycol) acrylate) Hydrogels. Gels, 9(1), 54. https://doi.org/10.3390/gels9010054