Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Method of Synthesis of ZnO NPs Gels
2.2. Investigation of Gels of ZnO NPs Modified with Polysaccharides
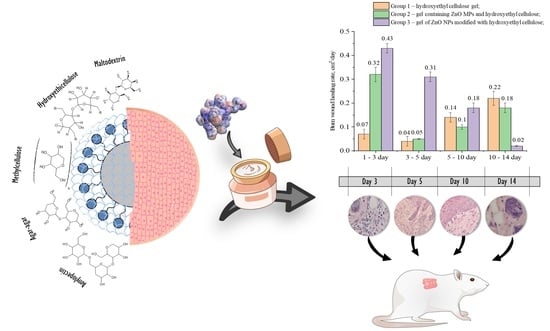
2.3. Approbation of a Gel of ZnO NPs Modified with Hydroxyethyl Cellulose
3. Conclusions
4. Materials and Methods
4.1. Substances and Reagents
4.2. Synthesis of ZnO NPs Gels
4.3. Synthesis of Gels of ZnO NPs Modified with Polysaccharides
4.4. Characteristics of ZnO NPs Gels
- -
- measurement range: from 400 to 4000 cm−1.
- -
- measurement step: 1 cm−1.
4.5. Investigation of the Effect of Synthesis Parameters on the Dynamic Viscosity of ZnO NPs Gels
- Molar concentration of zinc acetate, M;
- Active acidity of the reaction medium (pH);
- Duration of synthesis, minutes;
- Temperature of the reaction medium, ℃.
4.6. Investigation of Wound-Healing Ability of ZnO NPs Gels
4.7. Histological Research
- (1)
- A group treated with a gel of ZnO MPs and hydroxyethyl cellulose (control group);
- (2)
- A group treated with a gel of ZnO NPs modified with hydroxyethyl cellulose (experimental group).
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feijó, G.d.S.; Jantsch, J.; Correia, L.L.; Eller, S.; Furtado-Filho, O.V.; Giovenardi, M.; Porawski, M.; Braganhol, E.; Guedes, R.P. Neuroinflammatory Responses Following Zinc or Branched-Chain Amino Acids Supplementation in Obese Rats. Metab. Brain Dis. 2022, 37, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K. Essential Role of Zinc in Human Immunity: A Subject Review. Educ. Med. J. 2022, 1–9. [Google Scholar]
- Park, H.; Kim, W.; Kim, D.; Jeong, S.; Jung, Y. Mesalazine Activates Adenosine Monophosphate-Activated Protein Kinase: Implication in the Anti-Inflammatory Activity of This Anti-Colitic Drug. Curr. Mol. Pharmacol. 2019, 12, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwar, A.; Sharma, A. A Review on Role of Zinc as a Potent Immunity Boosting Agent. Mater. Today Proc. 2022, 68, 880–885. [Google Scholar] [CrossRef]
- Sheferov, I.; Balakireva, A.; Panteleev, D.; Spitskaya, I.; Orekhov, S.; Kazantsev, O.; Solovyeva, A.; Novopoltsev, D.; Melnikova, N. The Effect of Zinc Oxide Nanoparticles on Properties and Burn Wound Healing Activity of Thixotropic Xymedone Gels. Sci. Pharm. 2022, 90, 61. [Google Scholar] [CrossRef]
- Czyżowska, A.; Barbasz, A. A Review: Zinc Oxide Nanoparticles—Friends or Enemies? Int. J. Environ. Health Res. 2022, 32, 885–901. [Google Scholar] [CrossRef]
- Shafaee, H.; Khosropanah, H.; Rahimi, H.; Darroudi, M.; Rangrazi, A. Effects of Adding Cinnamon, ZnO, and CuO Nanoparticles on the Antibacterial Properties of a Glass Ionomer Cement as the Luting Agent for Orthodontic Bands and Their Cytotoxicity. J. Compos. Sci. 2022, 6, 336. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Staroń, A.; Długosz, O.; Domagała, D.; Janczyk, K.; Banach, M. Preparation of Zinc Oxide Nanoparticles Modified with Galactose and Assessment of Their Cytotoxic Properties. Appl. Phys. A 2022, 128, 387. [Google Scholar] [CrossRef]
- Song, H.; Lei, Y.; Xing, Z.; Liu, M. Minocycline plus Zinc Oxide Eugenol Cement Might Be A Promising Alternative for Acute Pulpitis. Evidence-Based Complement. Altern. Med. 2022, 2022, 1–5. [Google Scholar] [CrossRef]
- Faisal, S.; Abdullah; Rizwan, M.; Ullah, R.; Alotaibi, A.; Khattak, A.; Bibi, N.; Idrees, M. Paraclostridium Benzoelyticum Bacterium-Mediated Zinc Oxide Nanoparticles and Their In Vivo Multiple Biological Applications. Oxid. Med. Cell. Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO Size and Shape Effect on Antibacterial Activity and Cytotoxicity Profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO Nanoparticles Stabilized with Gelatin for Potential Use in Food Packaging Applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Swielam, E.M.; Atwa, N.A.; Agwa, M.M. Novel Design of Bandages Using Cotton Pads, Doped with Chitosan, Glycogen and ZnO Nanoparticles, Having Enhanced Antimicrobial and Wounds Healing Effects. Int. J. Biol. Macromol. 2022, 197, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Milan, P.B.; Amoupour, M. Enhanced Antimicrobial and Full-Thickness Wound Healing Efficiency of Hydrogels Loaded with Heparinized ZnO Nanoparticles: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2021, 166, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Le, V.A.T.; Trinh, T.X.; Chien, P.N.; Giang, N.N.; Zhang, X.-R.; Nam, S.-Y.; Heo, C.-Y. Evaluation of the Performance of a ZnO-Nanoparticle-Coated Hydrocolloid Patch in Wound Healing. Polymers (Basel) 2022, 14, 919. [Google Scholar] [CrossRef]
- Batool, M.; Khurshid, S.; Qureshi, Z.; Daoush, W.M. Adsorption, Antimicrobial and Wound Healing Activities of Biosynthesised Zinc Oxide Nanoparticles. Chem. Pap. 2021, 75, 893–907. [Google Scholar] [CrossRef]
- Ayala-Fuentes, J.C.; Chavez-Santoscoy, R.A. Nanotechnology as a Key to Enhance the Benefits and Improve the Bioavailability of Flavonoids in the Food Industry. Foods 2021, 10, 2701. [Google Scholar] [CrossRef]
- Mahajan, R.; Selim, A.; Neethu, K.M.; Sharma, S.; Shanmugam, V.; Jayamurugan, G. A Systematic Study to Unravel the Potential of Using Polysaccharides Based Organic-Nanoparticles versus Hybrid-Nanoparticles for Pesticide Delivery. Nanotechnology 2021, 32, 475704. [Google Scholar] [CrossRef]
- Hou, K.; Wang, Z. Application of Nanotechnology to Enhance Adsorption and Bioavailability of Procyanidins: A Review. Food Rev. Int. 2021, 1–15. [Google Scholar] [CrossRef]
- Asthana, N.; Pal, K.; Aljabali, A.A.A.; Tambuwala, M.M.; de Souza, F.G.; Pandey, K. Polyvinyl Alcohol (PVA) Mixed Green–Clay and Aloe Vera Based Polymeric Membrane Optimization: Peel-off Mask Formulation for Skin Care Cosmeceuticals in Green Nanotechnology. J. Mol. Struct. 2021, 1229, 129592. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, N.; Rastogi, M. Nanotechnology to Address Micronutrient and Macronutrient Deficiency. In Nanotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 35–39. ISBN 9781620819913. [Google Scholar]
- Mocioiu, O.-C.; Atkinson, I.; Mocioiu, A.-M.; Neagu, S.; Ruginescu, R.; Mitran, R.-A.; Enache, M. Effect of ZnO on Properties of Gels for Heritage Objects Conservation. Gels 2021, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, A.; Abbas Zadeh Haji Abadi, M.; Kianinia, Y.; Ghadiri, M. Critical Importance of PH and Collector Type on the Flotation of Sphalerite and Galena from a Low-Grade Lead–Zinc Ore. Sci. Rep. 2021, 11, 3103. [Google Scholar] [CrossRef]
- Olejnik, M.; Kersting, M.; Rosenkranz, N.; Loza, K.; Breisch, M.; Rostek, A.; Prymak, O.; Schürmeyer, L.; Westphal, G.; Köller, M.; et al. Cell-Biological Effects of Zinc Oxide Spheres and Rods from the Nano- to the Microscale at Sub-Toxic Levels. Cell Biol. Toxicol. 2021, 37, 573–593. [Google Scholar] [CrossRef]
- Kim, H.; Kwack, Y.-J.; Yun, E.-J.; Choi, W.-S. A Mixed Solution-Processed Gate Dielectric for Zinc-Tin Oxide Thin-Film Transistor and Its MIS Capacitance. Sci. Rep. 2016, 6, 33576. [Google Scholar] [CrossRef] [Green Version]
- Top, A.; Çetinkaya, H. Zinc Oxide and Zinc Hydroxide Formation via Aqueous Precipitation: Effect of the Preparation Route and Lysozyme Addition. Mater. Chem. Phys. 2015, 167, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials (Basel) 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Dey, K.; Agnelli, S.; Borsani, E.; Sartore, L. Degradation-Dependent Stress Relaxing Semi-Interpenetrating Networks of Hydroxyethyl Cellulose in Gelatin-PEG Hydrogel with Good Mechanical Stability and Reversibility. Gels 2021, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The Identification of Hydroxyl Groups on ZnO Nanoparticles by Infrared Spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092. [Google Scholar] [CrossRef]
- Preda, N.; Enculescu, M.; Zgura, I.; Socol, M.; Florica, C.; Evanghelidis, A.; Matei, E.; Enculescu, I. Zinc Oxide and Polysaccharides: Promising Candidates for Functional Nanomaterials. In Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2014; pp. 109–136. [Google Scholar]
- Sorbiun, M.; Shayegan Mehr, E.; Ramazani, A.; Taghavi Fardood, S. Green Synthesis of Zinc Oxide and Copper Oxide Nanoparticles Using Aqueous Extract of Oak Fruit Hull (Jaft) and Comparing Their Photocatalytic Degradation of Basic Violet 3. Int. J. Environ. Res. 2018, 12, 29–37. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kudo, H.; Suzuki, S.; Nemoto, N.; Sassa, S.; Sakamoto, S. Down Regulation by a Low-Zinc Diet in Gene Expression of Rat Prostatic Thymidylate Synthase and Thymidine Kinase. Nutr. Metab. (Lond) 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.S. Effects of Zinc Deficiency on Th1 and Th2 Cytokine Shifts. J. Infect. Dis. 2000, 182, S62–S68. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.; Mishra, S.; Verma, R.; Shikha, D. Synthesis of ZnO and CuO Nanoparticles via Sol Gel Method and Its Characterization by Using Various Technique. Discov. Mater. 2022, 2, 1. [Google Scholar] [CrossRef]
- Ismail, S.H.; Hamdy, A.; Ismail, T.A.; Mahboub, H.H.; Mahmoud, W.H.; Daoush, W.M. Synthesis and Characterization of Antibacterial Carbopol/ZnO Hybrid Nanoparticles Gel. Crystals 2021, 11, 1092. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-Gel Synthesis of Thorn-like ZnO Nanoparticles Endorsing Mechanical Stirring Effect and Their Antimicrobial Activities: Potential Role as Nano-Antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [Green Version]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Gouma, E.; Simos, Y.; Verginadis, I.; Lykoudis, E.; Evangelou, A.; Karkabounas, S. A Simple Procedure for Estimation of Total Body Surface Area and Determination of a New Value of Meeh’s Constant in Rats. Lab. Anim. 2012, 46, 40–45. [Google Scholar] [CrossRef]
- Babushkina, I.V.; Belova, S.V.; Gladkova, E.V.; Mamonova, I.A. Comparative Study of Complex Powdery Preparations for Soft Tissue Regeneration. Traumatol. Orthop. 2017, 13, 705–710. [Google Scholar]
- Blinov, A.V.; Nagdalian, A.A.; Povetkin, S.N.; Gvozdenko, A.A.; Verevkina, M.N.; Rzhepakovsky, I.V.; Lopteva, M.S.; Maglakelidze, D.G.; Kataeva, T.S.; Blinova, A.A.; et al. Surface-Oxidized Polymer-Stabilized Silver Nanoparticles as a Covering Component of Suture Materials. Micromachines 2022, 13, 1105. [Google Scholar] [CrossRef]



















| No. | a | b | c | d | Viscosity, MPa·s |
|---|---|---|---|---|---|
| 1 | 0.1 | 7.0 | 5 | 20 | 1.658 |
| 2 | 0.1 | 7.5 | 10 | 30 | 2.019 |
| 3 | 0.1 | 8.0 | 15 | 40 | 1.931 |
| 4 | 0.1 | 8.5 | 20 | 50 | 2.956 |
| 5 | 0.2 | 7.0 | 10 | 40 | 2.809 |
| 6 | 0.2 | 7.5 | 5 | 50 | 8.061 |
| 7 | 0.2 | 8.0 | 20 | 20 | 4.422 |
| 8 | 0.2 | 8.5 | 15 | 30 | 2.081 |
| 9 | 0.3 | 7.0 | 15 | 50 | 7.255 |
| 10 | 0.3 | 7.5 | 20 | 40 | 10.535 |
| 11 | 0.3 | 8.0 | 5 | 30 | 66.00 |
| 12 | 0.3 | 8.5 | 10 | 20 | 4.606 |
| 13 | 0.4 | 7.0 | 20 | 30 | 13.20 |
| 14 | 0.4 | 7.5 | 15 | 20 | 6.633 |
| 15 | 0.4 | 8 | 10 | 50 | 44.333 |
| 16 | 0.4 | 8.5 | 5 | 40 | 34.238 |
| Model | Energy, kcal/mol | HOMO, eV | LUMO, eV | ɳ, eV |
|---|---|---|---|---|
| ZnO-maltodextrin | −3140.336 | −0.262 | 0.097 | 0.1795 |
| ZnO-agar–agar | −2989.534 | −0.285 | 0.085 | 0.185 |
| ZnO-methylcellulose | −3260.501 | −0.263 | 0.106 | 0.1845 |
| ZnO-hydroxyethyl cellulose | −4210.149 | −0.297 | 0.089 | 0.193 |
| ZnO-amylopectin | −4354.377 | −0.249 | 0.119 | 0.184 |
| Λ,cm−1 | Bonds Fluctuations | λ,cm−1 | Bonds Fluctuations | λ,cm−1 | Bonds Fluctuations | λ,cm−1 | Bonds Fluctuations |
|---|---|---|---|---|---|---|---|
| Maltodextrin | ZnO-maltodextrin | Agar–agar | ZnO-agar–agar | ||||
| 3335 | C–H | 3399 | C–H | 3275 | C–H | 3414 | C–H |
| 2912 | C–H | 2880 | C–H | 3080 | C–H | 2893 | OH− |
| 1627 | C–C | 2899 | C–H | 2956 | C–H | 1564 | CH3 |
| 1437 | OH− | 1529 | C–C | 2916 | C–H | 1496 | OH− |
| 1388 | CH3 | 1401 | OH− | 1656 | C–C | 1342 | CH3 |
| 1211 | CH3 | 1296 | CH3 | 1462 | OH− | 1287 | CH3 |
| 1140 | CH2 | 978 | CH3 | 1415 | OH− | 1134 | CH2 |
| 1058 | CH2 | 883 | CH3 | 1332 | OH− | 1098 | CH2 |
| 989 | CH3 | 637 | C–H | 1263 | CH3 | 980 | CH3 |
| 918 | CH3 | 567 | Zn–O | 1159 | C–O | 875 | CH3 |
| 835 | CH3 | - | - | 1122 | C–O | 782 | C–H |
| 752 | C–H | - | - | 1084 | CH2 | 631 | C–H |
| 683 | C–H | - | - | 1030 | CH2 | 565 | Zn–O |
| - | - | - | - | 767 | CH2, CH3 | - | - |
| - | - | - | - | 729 | CH2, CH3 | - | - |
| Methylcellulose | ZnO-methylcellulose | Hydroxyethyl-cellulose | ZnO-hydroxyethylcellulose | ||||
| 3478 | C–H | 3109 | C–H | 3437 | C–H | 3239 | C–H |
| 3443 | C–H | 1919 | CH3 | 3283 | C–H | 2945 | C–H |
| 2940 | C–H | 1555 | C–C | 3167 | C–H | 1597 | C–C |
| 2916 | C–H | 1397 | OH− | 1649 | C–C | 1389 | OH− |
| 1680 | C=C | 1340 | CH3 | 1568 | CH2 | 1339 | CH3 |
| 1622 | C–C | 1288 | CH3 | 1410 | OH− | 1287 | CH3 |
| 1478 | CH3 | 1242 | CH3 | 1024 | CH3 | 1146 | CH3 |
| 1402 | OH− | 1003 | CH3 | 925 | CH3 | 1053 | CH3 |
| 1321 | CH3 | 974 | CH3 | 891 | CH3 | 1026 | CH3 |
| 1254 | CH3 | 886 | CH2 | 852 | CH2 | 936 | CH3 |
| 1180 | CH3 | 783 | C–H | 804 | CH2 | 829 | CH2 |
| 1134 | CH3 | 702 | C–H | - | - | 756 | C–H |
| 1057 | CH3 | 638 | C–H | - | - | 687 | C–H |
| 939 | CH3 | 569 | Zn–O | - | - | 615 | C–H |
| 885 | CH2 | 517 | CH2, CH3 | - | - | 558 | Zn–O |
| 685 | C–H | - | - | - | - | - | - |
| 661 | C–H | - | - | - | - | - | - |
| 561 | CH2, CH3 | - | - | - | - | - | - |
| 520 | CH2, CH3 | - | - | - | - | - | - |
| Amylopectin | ZnO-amylopectin | - | - | - | - | ||
| 3233 | C–H | 3404 | C–H | - | - | - | - |
| 2909 | C–H | 2874 | C–H | - | - | - | - |
| 1624 | C–C | 2800 | C–H | - | - | - | - |
| 1412 | OH− | 1554 | CH2 | - | - | - | - |
| 1331 | CH3 | 1383 | OH− | - | - | - | - |
| 1215 | CH3 | 1292 | CH3 | - | - | - | - |
| 1117 | CH3 | 1136 | CH3 | - | - | - | - |
| 1049 | CH3 | 1096 | CH3 | - | - | - | - |
| 1013 | CH3 | 978 | CH3 | - | - | - | - |
| 978 | CH3 | 886 | CH2 | - | - | - | - |
| 918 | CH3 | 785 | C–H | ||||
| 849 | CH2 | 740 | C–H | - | - | - | - |
| 716 | C–H | 625 | C–H | - | - | - | - |
| 661 | C–H | 565 | Zn–O | - | - | - | - |
| 569 | CH2, CH3 | - | - | - | - | - | - |
| 513 | CH2, CH3 | - | - | - | - | - | - |
| 417 | CH2, CH3 | - | - | - | - | - | - |
| Name of Parameters | Parameter Designation | Levels of Variable Variation | |||
|---|---|---|---|---|---|
| C((CH3COO)2Zn), M | x1 | 0.1 | 0.2 | 0.3 | 0.4 |
| pH | x2 | 7 | 7.5 | 8 | 8.5 |
| τ, minutes | x3 | 5 | 10 | 15 | 20 |
| t, °C | x4 | 20 | 30 | 40 | 50 |
| No. | Parameters | No. | Parameters | No. | Parameters |
|---|---|---|---|---|---|
| 1 | a1b1c1d1 | 7 | a2b3c4d1 | 13 | a4b1c4d2 |
| 2 | a1b2c2d2 | 8 | a2b4c3d2 | 14 | a4b2c3d1 |
| 3 | a1b3c3d3 | 9 | a3b1c3d4 | 15 | a4b3c2d4 |
| 4 | a1b4c4d4 | 10 | a3b2c4d3 | 16 | a4b4c1d3 |
| 5 | a2b1c2d3 | 11 | a3b3c1d2 | ||
| 6 | a2b2c1d4 | 12 | a3b4c2d1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagapetova, A.G.; et al. Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels 2023, 9, 57. https://doi.org/10.3390/gels9010057
Blinov AV, Kachanov MD, Gvozdenko AA, Nagdalian AA, Blinova AA, Rekhman ZA, Golik AB, Vakalov DS, Maglakelidze DG, Nagapetova AG, et al. Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels. 2023; 9(1):57. https://doi.org/10.3390/gels9010057
Chicago/Turabian StyleBlinov, Andrey V., Maksim D. Kachanov, Alexey A. Gvozdenko, Andrey A. Nagdalian, Anastasiya A. Blinova, Zafar A. Rekhman, Alexey B. Golik, Dmitriy S. Vakalov, David G. Maglakelidze, Anzhela G. Nagapetova, and et al. 2023. "Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels" Gels 9, no. 1: 57. https://doi.org/10.3390/gels9010057
APA StyleBlinov, A. V., Kachanov, M. D., Gvozdenko, A. A., Nagdalian, A. A., Blinova, A. A., Rekhman, Z. A., Golik, A. B., Vakalov, D. S., Maglakelidze, D. G., Nagapetova, A. G., Pokhilko, A. D., & Burkina, I. V. (2023). Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels, 9(1), 57. https://doi.org/10.3390/gels9010057