Forward Light Scattering of First to Third Generation Vitreous Body Replacement Hydrogels after Surgical Application Compared to Conventional Silicone Oils and Vitreous Body
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
- Chemical and viscoelastic properties of the tested hydrogels
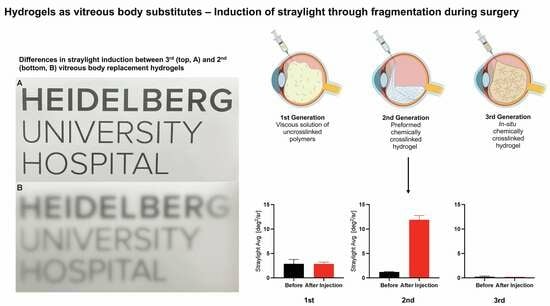
- All vitreous body replacements show low levels of stray light before fragmentation.
- Fragmentation induced by small-gauge injection greatly induces permanent stray light in the 2nd generation hydrogel but not in the 1st and 3rd generation hydrogels.
- Clinically used and novel vitreous body replacement strategies compared to the vitreous body.
2.2. Discussion
2.2.1. Summary
2.2.2. Forward Light Scattering
2.2.3. Reasons for the Increase in Stray Light after Gel Fragmentation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.1.1. Porcine and Human Vitreous Body Preparation
4.1.2. First Generation Hyaluronic Acid Gel
4.1.3. Second Generation Alginate Gel
4.1.4. Third Generation Tetra-PEG Gel
4.1.5. Measurement of forward Light-Scattering
4.1.6. Study Setup
4.1.7. Measurement of Viscosity and Viscoelastic Properties
4.1.8. Sol-Gel-Transition of the 3rd Generation Vitreous Body Replacement Hydrogel
4.1.9. pH-Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLeod, D. The Vitreous. Eye 1992, 6, vii–viii. [Google Scholar] [CrossRef]
- Sebag, J. Vitreous and Vision Degrading Myodesopsia. Prog. Retin. Eye Res. 2020, 79, 100847. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, D.A.; Skandalis, S.S.; Noulas, A.V.; Papageorgakopoulou, N.; Theocharis, A.D.; Karamanos, N.K. Hyaluronan and Chondroitin Sulfate Proteoglycans in the Supramolecular Organization of the Mammalian Vitreous Body. Connect. Tissue Res. 2008, 49, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, D.N. Vitrectomy for Vitreous Floaters: Analysis of the Benefits and Risks. Curr. Opin. Ophthalmol. 2015, 26, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.M.; Muni, R.H.; Nichani, P.; Kertes, P.J. Pars Plana Vitrectomy, Scleral Buckle, and Pneumatic Retinopexy for the Management of Rhegmatogenous Retinal Detachment: A Meta-Analysis. Surv. Ophthalmol. 2022, 67, 184–196. [Google Scholar] [CrossRef]
- Oellers, P.; Mahmoud, T. Surgery for Proliferative Diabetic Retinopathy: New Tips and Tricks. J. Ophthalmic Vis. Res. 2016, 11, 93. [Google Scholar] [CrossRef]
- Negretti, G.S.; Chan, W.; Pavesio, C.; Muqit, M.M.K. Vitrectomy for Endophthalmitis: 5-Year Study of Outcomes and Complications. BMJ Open Ophthalmol. 2020, 5, e000423. [Google Scholar] [CrossRef] [PubMed]
- Machemer, R.; Hickingbotham, D. The Three-Port Microcannular System for Closed Vitrectomy. Am. J. Ophthalmol. 1985, 100, 590–592. [Google Scholar] [CrossRef]
- Cibis, P.A.; Becker, B.; Okun, E.; Canaan, S. The Use of Liquid Silicone in Retinal Detachment Surgery. Arch. Ophthalmol. 1962, 68, 590–599. [Google Scholar] [CrossRef]
- Armaly, M.F. Ocular Tolerance to Silicones. I. Replacement of Aqueous and Vitreous by Silicone Fluids. Arch. Ophthalmol. 1962, 68, 390–395. [Google Scholar] [CrossRef]
- Nishi, K.; Nakamura, M.; Nishitsuka, K. Efficacy of Vitrectomy with Air Tamponade for Rhegmatogenous Retinal Detachment: A Prospective Study. Sci. Rep. 2023, 13, 10790. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Schickhardt, S.K.; Munro, D.; Khoramnia, R.; Scheuerle, A.; Mayer, C.S.; Auffarth, G.U. The Novel Use of High-Flow Polyimide Cannulas to Improve Silicone Oil Injectability in Vitreoretinal Surgery. Retina, 2022; Publish Ahead of Print. [Google Scholar] [CrossRef]
- Hammer, M.; Schickhardt, S.; Munro, D.; Scheuerle, A.; Khoramnia, R.; Uhl, P.; Auffarth, G.U. New Approaches to Explanting High-Viscosity Silicone Oil in Retinal Surgery—Polyimide Cannulas vs Extraction Sleeves vs a Luer-Trocar. Int. J. Retin. Vitr. 2023, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Schickhardt, S.; Munro, D.J.; Scheuerle, A.; Mayer, C.S.; Auffarth, G.U. Physicochemical Properties of Explanted Silicone Oil After Use as an Intraocular Tamponade. Transl. Vis. Sci. Technol. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Schickhardt, S.K.; Merz, P.R.; Khoramnia, R.; Scheuerle, A.F.; Mier, W.; Uhl, P.; Auffarth, G.U. Intravitreal Application: Physicochemical Properties of Drugs Dissolved in Silicone Oils of Different Density in Comparison to the Porcine Vitreous Body. Pharmaceutics 2022, 14, 1364. [Google Scholar] [CrossRef]
- Lu, Y.; Chan, Y.K.; Lau, L.H.; Wong, D.; Wong, J.K.W.; Shih, K.C.; Lai, S.M.; Shum, H.C. Amphiphilic Additives in Silicone Oil Tamponade and Emulsification: An Eye-on-a-chip Study. Acta Ophthalmol. 2020, 98, e232–e237. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Day, M.; Garvey, M.J.; English, R.; Wong, D. Increasing the Extensional Viscosity of Silicone Oil Reduces the Tendency for Emulsification. Retina 2010, 30, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Su, X.; Loh, X.J. Polymeric Hydrogels as a Vitreous Replacement Strategy in the Eye. Biomaterials 2021, 268, 120547. [Google Scholar] [CrossRef]
- Hayashi, K.; Okamoto, F.; Hoshi, S.; Katashima, T.; Zujur, D.C.; Li, X.; Shibayama, M.; Gilbert, E.P.; Chung, U.; Ohba, S.; et al. Fast-Forming Hydrogel with Ultralow Polymeric Content as an Artificial Vitreous Body. Nat. Biomed. Eng. 2017, 1, 0044. [Google Scholar] [CrossRef]
- Schnichels, S.; Schneider, N.; Hohenadl, C.; Hurst, J.; Schatz, A.; Januschowski, K.; Spitzer, M.S. Efficacy of Two Different Thiol-Modified Crosslinked Hyaluronate Formulations as Vitreous Replacement Compared to Silicone Oil in a Model of Retinal Detachment. PLoS ONE 2017, 12, e0172895. [Google Scholar] [CrossRef]
- Hurst, J.; Rickmann, A.; Heider, N.; Hohenadl, C.; Reither, C.; Schatz, A.; Schnichels, S.; Januschowski, K.; Spitzer, M.S. Long-Term Biocompatibility of a Highly Viscously Thiol-Modified Cross-Linked Hyaluronate as a Novel Vitreous Body Substitute. Front. Pharmacol. 2022, 13, 817353. [Google Scholar] [CrossRef]
- Januschowski, K.; Schnichels, S.; Hurst, J.; Hohenadl, C.; Reither, C.; Rickmann, A.; Pohl, L.; Bartz-Schmidt, K.-U.; Spitzer, M.S. Ex Vivo Biophysical Characterization of a Hydrogel-Based Artificial Vitreous Substitute. PLoS ONE 2019, 14, e0209217. [Google Scholar] [CrossRef]
- Wang, T.; Ran, R.; Ma, Y.; Zhang, M. Polymeric Hydrogel as a Vitreous Substitute: Current Research, Challenges, and Future Directions. Biomed. Mater. 2021, 16, 042012. [Google Scholar] [CrossRef]
- Calciu-Rusu, D.; Rothfuss, E.; Eckelt, J.; Haase, T.; Dick, H.B.; Wolf, B.A. Rheology of Sodium Hyaluronate Saline Solutions for Ophthalmic Use. Biomacromolecules 2007, 8, 1287–1292. [Google Scholar] [CrossRef]
- Baino, F. Towards an Ideal Biomaterial for Vitreous Replacement: Historical Overview and Future Trends. Acta Biomater. 2011, 7, 921–935. [Google Scholar] [CrossRef]
- Soman, N.; Banerjee, R. Artificial Vitreous Replacements. Biomed. Mater. Eng. 2003, 13, 59–74. [Google Scholar]
- Rainer, G. Intraocular Pressure Rise after Small Incision Cataract Surgery: A Randomised Intraindividual Comparison of Two Dispersive Viscoelastic Agents. Br. J. Ophthalmol. 2001, 85, 139–142. [Google Scholar] [CrossRef]
- Rainer, G.; Menapace, R.; Schmid, K.E.; Sacu, S.; Kiss, B.; Heinze, G.; Findl, O. Natural Course of Intraocular Pressure after Cataract Surgery with Sodium Chondroitin Sulfate 4%–Sodium Hyaluronate 3% (Viscoat). Ophthalmology 2005, 112, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Arshinoff, S.A. Dispersive-Cohesive Viscoelastic Soft Shell Technique. J. Cataract. Refract. Surg. 1999, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Poyer, J.F.; Chan, K.Y.; Arshinoff, S.A. Quantitative Method to Determine the Cohesion of Viscoelastic Agents by Dynamic Aspiration. J. Cataract. Refract. Surg. 1998, 24, 1130–1135. [Google Scholar] [CrossRef]
- Prinz, A.; Fennes, C.; Buehl, W.; Findl, O. Efficacy of Ophthalmic Viscosurgical Devices in Maintaining Corneal Epithelial Hydration and Clarity: In Vitro Assessment. J. Cataract. Refract. Surg. 2012, 38, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Januschowski, K.; Szurman, P. Novel Vitreous Substitutes: The next Frontier in Vitreoretinal Surgery. Curr. Opin. Ophthalmol. 2021, 32, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Rickmann, A.; Wahl, S.; Germann, A.; Stanzel, B.V.; Januschowski, K.; Szurman, P. Alginate- and Hyaluronic Acid–Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Schramm, C.; Spitzer, M.S.; Henke-Fahle, S.; Steinmetz, G.; Januschowski, K.; Heiduschka, P.; Geis-Gerstorfer, J.; Biedermann, T.; Bartz-Schmidt, K.U.; Szurman, P. The Cross-Linked Biopolymer Hyaluronic Acid as an Artificial Vitreous Substitute. Investig. Ophthalmol. Vis. Sci. 2012, 53, 613. [Google Scholar] [CrossRef]
- Charles, S.; Ho, A.C.; Dugel, P.U.; Riemann, C.D.; Berrocal, M.H.; Gupta, S.; Hamilton, C.; Abulon, D.J.K. Clinical Comparison of 27-Gauge and 23-Gauge Instruments on the Outcomes of Pars Plana Vitrectomy Surgery for the Treatment of Vitreoretinal Diseases. Curr. Opin. Ophthalmol. 2020, 31, 185–191. [Google Scholar] [CrossRef]
- Osawa, S.; Oshima, Y. 27-Gauge Vitrectomy. Dev. Ophthalmol. 2014, 54, 54–62. [Google Scholar] [CrossRef]
- Adamiec-Mroczek, J. 27-Gauge Sutureless Vitrectomy under Topical Anesthesia: A Pilot Study. Adv. Clin. Exp. Med. 2021, 30, 1099–1103. [Google Scholar] [CrossRef]
- Barth, H.; Crafoord, S.; Andréasson, S.; Ghosh, F. A Cross-Linked Hyaluronic Acid Hydrogel (Healaflow(®)) as a Novel Vitreous Substitute. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 697–703. [Google Scholar] [CrossRef]
- Barth, H.; Crafoord, S.; Ghosh, F. A New Retinal Detachment Treatment Model for Evaluation of Vitreous Tamponades. Curr. Eye Res. 2021, 46, 373–379. [Google Scholar] [CrossRef]
- Ren, X.J.; Bu, S.C.; Wu, D.; Liu, B.S.; Yang, F.H.; Hu, B.J.; Liu, J.P.; Zhang, X.M.; Dong, L.J.; Zheng, C.Z.; et al. Patching retinal breaks with healaflow in 27-gauge vitrectomy for the treatment of rhegmatogenous retinal detachment. Retina 2020, 40, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.K.; Jiang, P.; Torres-Flores, T.C.; Jacobs, K.M.; Chandler, H.L.; Swindle-Reilly, K.E. A Hydrogel Vitreous Substitute That Releases Antioxidant. Macromol. Biosci. 2020, 20, e1900305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Peng, Y.; Yang, C.; Liu, W.; Han, B. The Feasibility Study of an in Situ Marine Polysaccharide-Based Hydrogel as the Vitreous Substitute. J. Biomed. Mater. Res. A 2018, 106, 1997–2006. [Google Scholar] [CrossRef]
- Su, W.-Y.; Chen, K.-H.; Chen, Y.-C.; Lee, Y.-H.; Tseng, C.-L.; Lin, F.-H. An Injectable Oxidated Hyaluronic Acid/Adipic Acid Dihydrazide Hydrogel as a Vitreous Substitute. J. Biomater. Sci. Polym. Ed. 2011, 22, 1777–1797. [Google Scholar] [CrossRef]
- Yildirim, T.M.; Łabuz, G.; Hammer, M.; Son, H.-S.; Schickhardt, S.K.; Auffarth, G.U.; Khoramnia, R. A Novel Approach for Assessing Visual Impairment Caused by Intraocular Lens Opacification: High-Resolution Optical Coherence Tomography. Am. J. Ophthalmol. 2021, 226, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Vargas-Martín, F.; van den Berg, T.J.T.P.; López-Gil, N. Method for in Vitro Assessment of Straylight from Intraocular Lenses. Biomed. Opt. Express 2015, 6, 4457. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, T.J.T.P.; Franssen, L.; Kruijt, B.; Coppens, J.E. History of Ocular Straylight Measurement: A Review. Z. Med. Phys. 2013, 23, 6–20. [Google Scholar] [CrossRef]
- Łabuz, G.; Reus, N.J.; Van Den Berg, T.J.T.P. Straylight from Glistenings in Intraocular Lenses: In Vitro Study. J. Cataract. Refract. Surg. 2017, 43, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-S.; Łabuz, G.; Khoramnia, R.; Yildirim, T.M.; Choi, C.Y.; Knorz, M.C.; Auffarth, G.U. Visualization of Forward Light Scatter in Opacified Intraocular Lenses and Straylight Assessment. Diagnostics 2021, 11, 1512. [Google Scholar] [CrossRef]
- Castilla-Marti, M.; van den Berg, T.J.T.P.; de Smet, M.D. Effect of Vitreous Opacities on Straylight Measurements. Retina 2015, 35, 1240–1246. [Google Scholar] [CrossRef]
- Filas, B.A.; Zhang, Q.; Okamoto, R.J.; Shui, Y.-B.; Beebe, D.C. Enzymatic Degradation Identifies Components Responsible for the Structural Properties of the Vitreous Body. Investig. Ophthalmol. Vis. Sci. 2014, 55, 55. [Google Scholar] [CrossRef]
- Schulz, A.; Wahl, S.; Rickmann, A.; Ludwig, J.; Stanzel, B.V.; von Briesen, H.; Szurman, P. Age-Related Loss of Human Vitreal Viscoelasticity. Transl. Vis. Sci. Technol. 2019, 8, 56. [Google Scholar] [CrossRef]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Borkenstein, A.F.; Borkenstein, E.-M.; Malyugin, B. Ophthalmic Viscosurgical Devices (OVDs) in Challenging Cases: A Review. Ophthalmol. Ther. 2021, 10, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of Cross-Linking with Calcium Ions on the Physical Properties of Alginate Films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Pounder, R.J.; Stanford, M.J.; Brooks, P.; Richards, S.P.; Dove, A.P. Metal Free Thiol–Maleimide ‘Click’ Reaction as a Mild Functionalisation Strategy for Degradable Polymers. Chem. Commun. 2008, 5158. [Google Scholar] [CrossRef]







| Name | Monomer/Compounds | Mechanism of Gelation | Reason for Use |
|---|---|---|---|
| G1: Hyaluronic Acid 1% | High molecular weight Hyaluronic Acid (1.200–2.000 kDa) | Only monomers, no gelation | Clinically used in anterior segment surgery |
| G2: Crosslinked Alginate | Alginate (1000 kDa) 11.6 mM calcium sulfate dihydrate solution | Crosslinked by complexing alginate via Ca2+ | One of the first G2 strategies, well-characterized |
| G3: Oligo-Tetra-PEG | Tetra-PEG functionalized with thiol and maleimide functional groups | Crosslinked via click-chemistry of different oligomers | Remained clear for one year in rabbit eyes without toxicity |
| Siluron 5000 | 100% Polydimethylsiloxane | Only monomers, no gelation | Currently clinically used |
| Densiron 68 | 30.5% F6H8, 69.5% Polydimethylsiloxane | Only monomers, no gelation | Currently clinically used |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammer, M.; Herth, J.; Muuss, M.; Schickhardt, S.; Scheuerle, A.; Khoramnia, R.; Łabuz, G.; Uhl, P.; Auffarth, G.U. Forward Light Scattering of First to Third Generation Vitreous Body Replacement Hydrogels after Surgical Application Compared to Conventional Silicone Oils and Vitreous Body. Gels 2023, 9, 837. https://doi.org/10.3390/gels9100837
Hammer M, Herth J, Muuss M, Schickhardt S, Scheuerle A, Khoramnia R, Łabuz G, Uhl P, Auffarth GU. Forward Light Scattering of First to Third Generation Vitreous Body Replacement Hydrogels after Surgical Application Compared to Conventional Silicone Oils and Vitreous Body. Gels. 2023; 9(10):837. https://doi.org/10.3390/gels9100837
Chicago/Turabian StyleHammer, Maximilian, Jonathan Herth, Marcel Muuss, Sonja Schickhardt, Alexander Scheuerle, Ramin Khoramnia, Grzegorz Łabuz, Philipp Uhl, and Gerd Uwe Auffarth. 2023. "Forward Light Scattering of First to Third Generation Vitreous Body Replacement Hydrogels after Surgical Application Compared to Conventional Silicone Oils and Vitreous Body" Gels 9, no. 10: 837. https://doi.org/10.3390/gels9100837
APA StyleHammer, M., Herth, J., Muuss, M., Schickhardt, S., Scheuerle, A., Khoramnia, R., Łabuz, G., Uhl, P., & Auffarth, G. U. (2023). Forward Light Scattering of First to Third Generation Vitreous Body Replacement Hydrogels after Surgical Application Compared to Conventional Silicone Oils and Vitreous Body. Gels, 9(10), 837. https://doi.org/10.3390/gels9100837