Superior Conductivity of Transparent ZnO/MoS2 Composite Films for Optoelectronic and Solar Cell Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optical Properties of ZnO/MoS2 Composite Films and Dispersion Calculations
2.2. Crystallographic Properties of ZnO/MoS2 Composite Films
2.3. SEM, TEM/EDS, and AFM Measurements of ZnO/MoS2 Composite Film
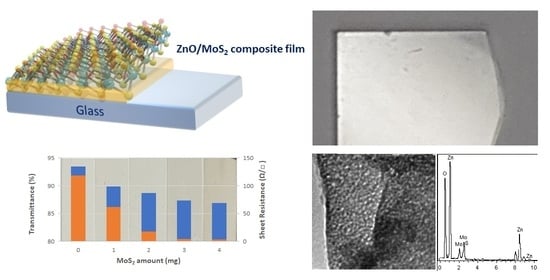
2.4. Sheet Resistance Measurements of ZnO/MoS2 Composite Films
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.; Cameron, D. Optical and electrical properties of transparent conductive ITO thin films deposited by sol–gel process. Thin Solid Films 2000, 377–378, 455–459. [Google Scholar] [CrossRef]
- Mokhtar, H.; Faouzi, G.; Lassaad, E. Characterization of ITO Thin Films Prepared by Sol-gel Spin-Coating Technique. Sens. Transducers 2014, 27, 198. [Google Scholar]
- Smith, A.; Laurent, J.M.; Smith, D.S.; Bonnet, J.P.; Clemente, R.R. Relation between solution chemistry and morphology of SnO2-based thin films deposited by a pyrosol process. Thin Solid Films 1995, 266, 20–30. [Google Scholar] [CrossRef]
- Adedokun, O. Review on Transparent Conductive Oxides Thin Films deposited by Sol-gel spin coating technique. Int. J. Eng. Sci. Appl. 2018, 2, 88–97. [Google Scholar]
- Dawood, Y.Z.; Hassoni, M.H.; Mohamad, M.S. Effect of Solution Concentration on Some Optical Properties of Indium Oxide Doped with SnO2 Thin Films Prepared by Chemical Spray Pyrolysis Technique. Int. J. Pure Appl. Phys. 2014, 2, 1–6. [Google Scholar]
- Moradi-Haji Jafan, M.; Zamani-Meymian, M.R.; Rahimi, R.; Rabbani, M. The effect of solvents and the thickness on structural, optical and electrical properties of ITO thin films prepared by a sol–gel spin-coating process. J. Nanostruct. Chem. 2014, 4, 89. [Google Scholar] [CrossRef]
- Hsu, H.; Lin, D.; Lu, G.; Ko, T.; Chen, H. Optical and electrical transport properties of ZnO/MoS2 heterojunction p-n structure. Mater. Chem. Phys. 2018, 220, 433–440. [Google Scholar] [CrossRef]
- Lu, J.G.; Fujita, S.; Kawaharamura, T.; Nishinaka, H.; Kamada, Y.; Ohshima, T.; Ye, Z.Z.; Zeng, Y.J.; Zhang, Y.Z.; Zhu, L.P.; et al. Carrier concentration dependence of band gap shift in n -type ZnO:Al films. J. Appl. Phys. 2007, 101, 083705. [Google Scholar] [CrossRef]
- Burstein, E. Anomalous optical absorption limit in InSb. Phys. Rev. 1954, 93, 632–633. [Google Scholar] [CrossRef]
- Moss, T.S. The Interpretation of the Properties of Indium Antimonide. Proc. Phys. Soc. Sect. B 1954, 67, 775–782. [Google Scholar] [CrossRef]
- Park, S.-M.; Ikegami, T.; Ebihara, K. Investigation of transparent conductive oxide Al-doped ZnO films produced by pulsed laser deposition. Jpn. J. Appl. Phys. 2005, 44, 8027. [Google Scholar] [CrossRef]
- Agura, H.; Suzuki, A.; Matsushita, T.; Aoki, T.; Okuda, M. Low resistivity transparent conducting Al-doped ZnO films prepared by pulsed laser deposition. Thin Solid Films 2003, 445, 263. [Google Scholar] [CrossRef]
- Kang, J.-W.; Jeong, W.-I.; Kim, J.-J.; Kim, H.-K.; Kim, D.-G.; Lee, G.-H. High-Performance Flexible Organic Light-Emitting Diodes Using Amorphous Indium Zinc Oxide Anode. Electrochem. Solid-State Lett. 2007, 10, J75. [Google Scholar] [CrossRef]
- Park, Y.-S.; Kim, H.-K. Flexible indium zinc oxide/Ag/indium zinc oxide multilayer electrode grown on polyethersulfone substrate by cost-efficient roll-to-roll sputtering for flexible organic photovoltaics. J. Vac. Sci. Technol. A Vac. Surf. Films 2010, 28, 41–47. [Google Scholar] [CrossRef]
- Gonçalves, G.; Grasso, V.; Barquinha, P.; Pereira, L.; Elamurugu, E.; Brignone, M.; Martins, R.; Lambertini, V.; Fortunato, E. Role of room temperature sputtered high conductive and high transparent indium zinc oxide film contacts on the performance of orange, green, and blue organic light emitting diodes. Plasma Process. Polym. 2011, 8, 340–345. [Google Scholar] [CrossRef]
- Arca, E.; Fleischer, K.; Shvets, I.V. An alternative fluorine precursor for the synthesis of SnO2:F by spray pyrolysis. Thin Solid Films 2012, 520, 1856–1861. [Google Scholar] [CrossRef]
- De La Garza-Guadarrama, V.; Sanchez-Juarez, A.; Tiburcio-Silver, A.; Ortiz, A. Growth and characterization of SnOx: F thin films prepared by pyrolysis of SnCl2. J. Mater. Sci. Lett. 2001, 20, 219–221. [Google Scholar] [CrossRef]
- Shin, S.W.; Pawa, S.M.; Kim, T.W.; Moon, J.H.; Kim, J.H. Effect of film thickness on the structural and electrical properties of Ga-doped ZnO thin films prepared on glass and Al2O3 (0001) substrates by RF magnetron sputtering method. J. Mater. Res. 2009, 24, 441–447. [Google Scholar] [CrossRef]
- Clatot, J.; Campet, G.; Zeinert, A.; Labrugère, C.; Nistor, M.; Rougier, A. Low temperature Si doped ZnO thin films for transparent conducting oxides. Sol. Energy Mater. Sol. Cells 2011, 95, 2357–2362. [Google Scholar] [CrossRef]
- Kim, M.S.; Yim, K.G.; Jeong-Sik, S.; Jae-Young, L. Effects of Al Concentration on Structural and Optical Properties of Al-doped ZnO Thin Films”, Structural and Optical Properties of AZO. Thin Films Bull. Korean Chem. Soc. 2012, 33, 1235. [Google Scholar] [CrossRef]
- Luo, L.; Rossell, M.D.; Xie, D.; Erni, R.; Niederberger, M. Microwave-assisted nonaqueous Sol-Gel synthesis: From Al:ZnO nanoparticles to transparent conducting films. ACS Sustain. Chem. Eng. 2013, 1, 152–160. [Google Scholar] [CrossRef]
- Yildirim, M.A.; Ates, A.; Astam, A. Annealing and light effect on structural, optical and electrical properties of CuS, CuZnS and ZnS thin films grown by the SILAR method. Phys. E Lowdimens. Syst. Nanostruct. 2009, 41, 1365–1372. [Google Scholar] [CrossRef]
- Tan, Y.; Resasco, D.E. Dispersion of Single-Walled Carbon Nanotubes of Narrow Diameter Distribution. J. Phys. Chem. B 2005, 109, 14454–14460. [Google Scholar] [CrossRef] [PubMed]
- Düzkan, B.; Özuğur Uysal, B.; Pekcan, Ö. Surfactant-free One-step Fabrication of Gelatin/PAAm/MWCNT Composites for Biomedical Applications. Polym. Bull. 2021, 52, 1597–1614. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. The Reflexion of X-rays by Crystals. Proc. R. Soc. Lond. A 1913, 88, 428–438. [Google Scholar]
- Scherrer, P. Bestimmung der Grösse und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen 1918, 26, 98–100. [Google Scholar]
- Nath, D.; Singh, F.; Das, R. X-ray Diffraction Analysis by Williamson-Hall, Halder-Wagner and Size-Strain Plot Methods of CdSe Nanoparticles- A comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD) Official Web Site. Available online: https://www.icdd.com/?gclid=Cj0KCQiA6t6ABhDMARIsAONIYyyW3wt2WBi-PQdQmnh5tYvXaV3cA265VNLLYEyj5x2DtBWPO9K33EwaAs91EALw_wcB (accessed on 25 June 2023).
- Materials Project Online Platform. Available online: https://materialsproject.org/materials/mp-2133/ (accessed on 25 June 2023).
- Rusu, D.I.; Rusu, G.G.; Luca, D. Structural Characteristics and Optical Properties of Thermally Oxidized Zinc Films. Acta Phys. Pol. A 2011, 119, 850–856. [Google Scholar] [CrossRef]
- Gu, F.; Wang, S.F.; Lü, M.K.; Zhou, G.J.; Xu, D.; Yuan, D.R. Structure Evaluation and Highly Enhanced Luminescence of Dy3+-Doped ZnO Nanocrystals by Li+Doping via Combustion Method. Langmuir 2004, 20, 3528–3531. [Google Scholar] [CrossRef]
- Prakash, T.; Jayaprakash, R.; Neri, G.; Kumar, S. Synthesis of ZnO Nanostructures by Microwave Irradiation Using Albumen as a Template. J. Nanoparticles 2013, 2013, 274894. [Google Scholar] [CrossRef]
- Singh, A.; Vishwakarma, H.L. Study of structural, morphological, optical and electroluminescent properties of undoped ZnO nanorods grown by a simple chemical precipitation. Mater. Sci.-Pol. 2015, 33, 751–759. [Google Scholar] [CrossRef]
- Singh, S.; Chakrabarti, P. Comparison of the structural and optical properties of ZnO thin films deposited by three different methods for optoelectronic applications. Superlattices Microstruct. 2013, 64, 283–293. [Google Scholar] [CrossRef]
- Veer, D.; Singh, R.M.; Kumar, H. Structural and Optical Characterization of ZnO-TiO2-SiO2 Nanocomposites Synthesized by Sol-Gel Technique. Asian J. Chem. 2017, 29, 2391–2395. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Kim, A.; Won, Y. Highly Transparent Low Resistance ZnO/Ag Nanowire/ZnO Composite Electrode for Thin Film Solar Cells. ACS Nano 2013, 7, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Tonny, K.N.; Rafique, R.; Sharmin, A.; Bashar, M.S.; Mahmood, Z.H. Electrical, optical and structural properties of transparent conducting Al doped ZnO (AZO) deposited by sol-gel spin coating. AIP Adv. 2018, 8, 065307. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zeng, H. ZnO-Based Transparent Conductive Thin Films: Doping, Performance’, and Processing. J. Nanomater. 2013, 2013, 196521. [Google Scholar] [CrossRef]
- Ali, D.; Butt, M.Z.; Coughlan, C.; Caffrey, D.; Shvets, I.V.; Fleischer, K. Nitrogen grain-boundary passivation of In-doped ZnO transparent conducting oxide. Phys. Rev. Mater. 2018, 2, 043402. [Google Scholar] [CrossRef]
- Jiamprasertboon, A.; Dixon, S.; Sathasivam, S.; Powell, M.; Lu, Y.; Siritanon, T.; Carmalt, C. Low-Cost One-Step Fabrication of Highly Conductive ZnO:Cl Transparent Thin Films with Tunable Photocatalytic Properties via Aerosol-Assisted Chemical Vapor Deposition. ACS Appl. Electron. Mater. 2019, 1, 1408–1417. [Google Scholar] [CrossRef]
- Luo, Y.-T.; Zhou, Z.-H.; Huang, Z.-B.; Juang, J.-Y.; Xu, L. Facile and versatile fabrication process for AgNW/GZO transparent composite electrodes for photovoltaic applications by atmospheric pressure plasma jet. Appl. Surf. Sci. 2023, 635, 157767. [Google Scholar] [CrossRef]
- Rashmi, N.; Serrao, F.J.; Kindalkar, V.S.; Kumara, K.; Rithin Kumar, N.B.; Savitha, M.B.; D’sa Joyline, G. Investigations on structural, optical and electrical properties of microwave-assisted rGO:ZnO nanocomposite thin films. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Safdar, H.; Aydın, R.; Şahin, B. Syntheses, structural evolution, electrical and optoelectronic characterization of ZnO/CuO composite films doped with transition metal Mn2+ ions. Ceram. Int. 2022, 48, 26678–26688. [Google Scholar] [CrossRef]
- Ganesh, V.N.; Anila, K.R.; Jayarama, A.; Bhat, S.; Shantharama Rai, C.; Pinto, R. Spray pyrolysis deposited aluminium-indium zinc oxide thin films and study of their electrical and photoluminescence properties. Mater. Today Proc. 2022, 55, 148–154. [Google Scholar] [CrossRef]















| Sample with Doping Amount of MoS2 in mg | Area of Resonant Band | Area of Nonresonant Background | Resonance Ratio | Width of Resonant Band (nm) | Height of Resonant Band (au) | Normalized Width (nm/au) |
|---|---|---|---|---|---|---|
| 0 | 14.0664 | 2.7493 | 5.1164 | 113 | 0.1546 | 730.9185 |
| 1 | 90.7485 | 20.1200 | 4.5104 | 160 | 0.1714 | 933.2439 |
| 2 | 101.9256 | 21.6664 | 4.7043 | 160 | 0.2025 | 790.0064 |
| 3 | 116.8828 | 18.3885 | 6.3563 | 86 | 0.2250 | 382.1859 |
| 4 | 104.6548 | 10.0995 | 10.3624 | 50 | 0.2354 | 212.4104 |
| Peak Position | FWHM | Crystallite Size | Calculated from Bragg’s Law | ICDD Theoretical Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2θ (°) | β hkl (°) | L (nm) | d (Å) | hkl | 2θ | d (Å) | 4sinθ | β cos θ | |
| 31.556 | 0.699 | 21.545 | 2.835 | 100 | 31.405 | 2.848 | 0.455 | 1.088 | 0.012 |
| 34.186 | 0.702 | 21.609 | 2.623 | 002 | 33.780 | 2.653 | 1.139 | 1.176 | 0.012 |
| 36.222 | 0.696 | 21.897 | 2.480 | 101 | 35.777 | 2.510 | 1.200 | 1.243 | 0.012 |
| 47.192 | 0.691 | 22.880 | 1.926 | 102 | 46.790 | 1.942 | 0.831 | 1.601 | 0.011 |
| 56.433 | 0.694 | 23.688 | 1.630 | 110 | 55.909 | 1.645 | 0.884 | 1.891 | 0.011 |
| 62.724 | 0.699 | 24.276 | 1.481 | 103 | 61.729 | 1.503 | 1.449 | 2.082 | 0.010 |
| 66.105 | 0.698 | 24.756 | 1.413 | 200 | 65.543 | 1.424 | 0.743 | 2.182 | 0.010 |
| 67.493 | 0.706 | 24.692 | 1.388 | 112 | 66.941 | 1.398 | 0.737 | 2.222 | 0.010 |
| 68.707 | 0.713 | 24.637 | 1.366 | 201 | 68.173 | 1.376 | 0.719 | 2.257 | 0.010 |
| Lavg = 23.331 nm | |||||||||
| MoS2 Amount (mg) | Film Thickness (nm) | Sheet Resistance (Ω/□) | Transmittance (%) at 550 nm | Figure of Merit (FoM) |
|---|---|---|---|---|
| 0 | 423 ± 2 | 118.112 | 93.464 | 4.31 × 10−3 |
| 1 | 393 ± 1 | 62.014 | 89.860 | 5.54 × 10−3 |
| 2 | 412 ± 4 | 17.857 | 88.715 | 1.69 × 10−2 |
| 3 | 423 ± 2 | 4.358 | 87.381 | 5.96 × 10−2 |
| 4 | 414 ± 3 | 2.842 | 86.907 | 8.65 × 10−2 |
| Material | Deposition Method | Sheet Resistance (Ω/□) | Transmittance (%) | Figure of Merit (FoM) | Reference |
|---|---|---|---|---|---|
| AgZnO (silver nanowire zinc oxide) | Sol–gel | 8 | 91 | 4.87 × 10−2 | [38] |
| AZO (aluminum zinc oxide) | Sol–gel | 148.43 | 66.23 | 1.09 × 10−4 | [39] |
| Ga/ZnO (gallium zinc oxide) | PLD | 40.6 | 90 | 8.59 × 10−3 | [40] |
| In/ZnO (indium zinc oxide) | PLD | 42.3 | 85 | 4.65 × 10−3 | [40] |
| F/ZnO (iron zinc oxide) | PLD | 24.15 | 90 | 1.44 × 10−2 | [40] |
| Si/ZnO (silicon zinc oxide) | PLD | 41.33 | 80 | 2.60 × 10−3 | [40] |
| In/ZnO (indium zinc oxide) | Spray pyrolysis | 32 | 90 | 1.09 × 10−2 | [41] |
| Cl/ZnO (chlorine zinc oxide) | CVD | 41.9 | 80 | 2.56 × 10−3 | [42] |
| AgNW/GZO (silver nanowire gallium zinc oxide) | Atmospheric pressure plasma jet | 68.3 | 7.3 | 3.03 × 10−3 | [43] |
| rGO/ZnO (reduced graphene oxide zinc oxide) | Microwave-assisted sol–gel | 80 | 3.24 | 3.31 × 10−2 | [44] |
| CuO/ZnO (copper oxide zinc oxide) | Successive ionic layer adsorption and reaction (SILAR) | 20 | 0.41 | 2.70 × 10−7 | [45] |
| AIZO (aluminum indium zinc oxide) | Spray pyrolysis | 85.8 | 25.5 | 8.48 × 10−3 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tareq, S.; Kirkil, G.; Özuğur Uysal, B. Superior Conductivity of Transparent ZnO/MoS2 Composite Films for Optoelectronic and Solar Cell Applications. Gels 2023, 9, 906. https://doi.org/10.3390/gels9110906
Tareq S, Kirkil G, Özuğur Uysal B. Superior Conductivity of Transparent ZnO/MoS2 Composite Films for Optoelectronic and Solar Cell Applications. Gels. 2023; 9(11):906. https://doi.org/10.3390/gels9110906
Chicago/Turabian StyleTareq, Shahad, Gokhan Kirkil, and Bengü Özuğur Uysal. 2023. "Superior Conductivity of Transparent ZnO/MoS2 Composite Films for Optoelectronic and Solar Cell Applications" Gels 9, no. 11: 906. https://doi.org/10.3390/gels9110906
APA StyleTareq, S., Kirkil, G., & Özuğur Uysal, B. (2023). Superior Conductivity of Transparent ZnO/MoS2 Composite Films for Optoelectronic and Solar Cell Applications. Gels, 9(11), 906. https://doi.org/10.3390/gels9110906