Hard-to-Heal Wound Healing: Superiority of Hydrogel EHO-85 (Containing Olea europaea Leaf Extract) vs. a Standard Hydrogel. A Randomized Controlled Trial
Abstract
:1. Introduction
2. Results and Discussion
2.1. Description of the Study Population and Typology of Hard-to-Heal Wounds
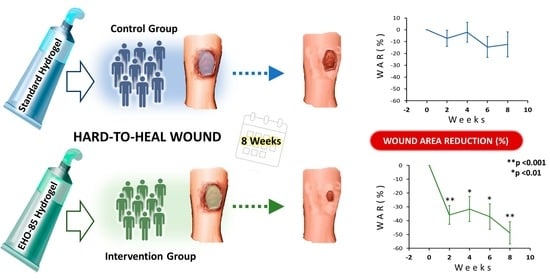
2.2. Wound-Area Reduction and Healing Rate
3. Conclusions
4. Materials and Methods
4.1. Study Design
4.2. Participants and Procedures
4.3. Randomization and Stratification
4.4. Outcomes
4.5. Statistical Analyses
4.6. Analyses of Efficacy Results
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guest, J.F.; Vowden, K.; Vowden, P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J. Wound Care 2017, 26, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Prim. 2022, 8, 50. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef]
- Salenius, J.E.; Suntila, M.; Ahti, T.; Huhtala, H.; Vaalasti, A.; Salmi, T.T.; Kimpimäki, T. Long-term mortality among patients with chronic ulcers. Acta Derm. Venereol. 2021, 101, adv00455. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Milne, J.; Searle, R.; Styche, T. The characteristics and impact of hard-to-heal wounds: Results of a standardised survey. J. Wound Care 2020, 29, 282–288. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Westby, M.J.; Dumville, J.C.; Soares, M.O.; Stubbs, N.; Norman, G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 6, CD011947. [Google Scholar] [CrossRef]
- Ribeiro, C.T.D.; Dias, F.A.L.; Fregonezi, G.A.F. Hydrogel dressings for venous leg ulcers. Cochrane database Syst. Rev. 2022, 8, CD010738. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2020, 236, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Goossens, A.; Cleenewerck, M.B. New wound dressings: Classification, tolerance. Eur. J. Dermatol. 2010, 20, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Vogel, P.; Machado, I.K.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Dal Bosco, S.M. Beneficios polifenoles hoja de olivo (Olea europaea L.) para la salud humana. Nutr. Hosp. 2015, 31, 1427–1433. [Google Scholar] [CrossRef]
- Casado-Diaz, A.; Moreno-Rojas, J.M.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Tunez, I.; Torre, M.L.; Pérez, M.B.; Priego-Capote, F.; Pereira-Caro, G. Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract. Pharmaceutics 2022, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; La Torre, M.; Priego-Capote, F.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Pérez, M.B.; Tunez, I. EHO-85: A Multifunctional Amorphous Hydrogel for Wound Healing Containing Olea europaea Leaf Extract: Effects on Wound Microenvironment and Preclinical Evaluation. J. Clin. Med. 2022, 11, 1229. [Google Scholar] [CrossRef]
- Verdú-soriano, J.; de Cristino-Espinar, M.; Luna-morales, S.; Dios-guerra, C.; Caballero-villarraso, J.; Moreno-moreno, P.; Casado-díaz, A.; Berenguer-pérez, M.; Guler-caamaño, I.; Laosa-Zafra, O.; et al. Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial. J. Clin. Med. 2022, 11, 1260. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Kapp, S.; Miller, C.; Santamaria, N. The quality of life of people who have chronic wounds and who self-treat. J. Clin. Nurs. 2018, 27, 182–192. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Opt Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Nagoba, B.S.; Suryawanshi, N.M.; Wadher, B.; Selkar, S. Acidic environment and wound healing: A review. Wounds 2015, 27, 5–11. [Google Scholar]
- Greener, B.; Hughes, A.A.; Bannister, N.P.; Douglass, J. Proteases and pH in chronic wounds. J. Wound Care 2005, 14, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Wimpenny, J.W.T.; Davis, J.G. Effect of three preservatives on the growth of Bacillus cereus, Vero cytotoxigenic Escherichia coli and Staphylococcus aureus, on plates with gradients of pH and sodium chloride concentration. Int. J. Food Microbiol. 1993, 17, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.; Strudwick, X.L.; Song, Y.M.; Cowin, A.J.; Garg, S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef]
- Younis, I. Role of oxygen in wound healing. J. Wound Care 2020, 29, S4–S10. [Google Scholar] [CrossRef]
- Leveen, H.H.; Falk, G.; Borek, B.; Diaz, C.; Lynfield, Y.; Wynkoop, B.J.; Mabunda, G.A.; Rubricius, J.L.; Christoudias, G.C. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann. Surg. 1973, 178, 745–753. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Carels, C.E.; Lundvig, D.M.S. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef] [PubMed]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Mehraein, F.; Sarbishegi, M.; Aslani, A. Evaluation of effect of oleuropein on skin wound healing in aged male BALB/c mice. Cell J. 2014, 16, 25–30. [Google Scholar] [PubMed]
- Dumville, J.C.; Stubbs, N.; Keogh, S.J.; Walker, R.M.; Liu, Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 2015, CD011226. [Google Scholar] [CrossRef]
- Humbert, P.; Faivre, B.; Véran, Y.; Debure, C.; Truchetet, F.; Bécherel, P.A.; Plantin, P.; Kerihuel, J.C.; Eming, S.A.; Dissemond, J.; et al. Protease-modulating polyacrylate-based hydrogel stimulates wound bed preparation in venous leg ulcers—A randomized controlled trial. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1742–1750. [Google Scholar] [CrossRef]
- Edmonds, M.; Lázaro-Martínez, J.L.; Alfayate-García, J.M.; Martini, J.; Petit, J.M.; Rayman, G.; Lobmann, R.; Uccioli, L.; Sauvadet, A.; Bohbot, S.; et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): An international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 186–196. [Google Scholar] [CrossRef]
- Meaume, S.; Truchetet, F.; Cambazard, F.; Lok, C.; Debure, C.; Dalac, S.; Lazareth, I.; Sigal, M.L.; Sauvadet, A.; Bohbot, S.; et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012, 20, 500–511. [Google Scholar] [CrossRef]
- Verdú-Soriano, J.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G.; Berenguer-Pérez, M.; Vílchez, S.; Esquena, J.; et al. EHO-85, Novel Amorphous Antioxidant Hydrogel, Containing Olea europaea Leaf Extract-Rheological Properties, and Superiority over a Standard Hydrogel in Accelerating Early Wound Healing: A Randomized Controlled Trial. Pharmaceutics 2023, 15, 1925. [Google Scholar] [CrossRef]
- Dereure, O.; Mikosinki, J.; Zegota, Z.; Allaert, F.A. RCT to evaluate a hyaluronic acid containing gauze pad in leg ulcers of venous or mixed aetiology. J. Wound Care 2012, 21, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, M.; Eisenbud, D.E.; Phillips, T.; Harding, K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008, 16, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Gelfand, J.M.; Hoffstad, O.; Berlin, J.A. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003, 26, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, E.S.; Zubkov, L.; Mao, X.; Neidrauer, M.; Rannou, N.; Weingarten, M.S. Image analysis of chronic wounds for determining the surface area. Wound Repair Regen. 2010, 18, 349–358. [Google Scholar] [CrossRef]
- Meaume, S.; Ourabah, Z.; Romanelli, M.; Manopulo, R.; De Vathaire, F.; Salomon, D.; Saura, J.H. Efficacy and tolerance of a hydrocolloid dressing containing hyaluronic acid for the treatment of leg ulcers of venous or mixed origin. Curr. Med. Res. Opin. 2008, 24, 2729–2739. [Google Scholar] [CrossRef]
- Ortonne, J.P. A controlled study of the activity of hyaluronic acid in the treatment of venous leg ulcers. J. Dermatolog. Treat. 1996, 7, 75–81. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Hoffstad, O.; Margolis, D.J. Surrogate endpoints for the treatment of venous leg ulcers. J. Invest. Dermatol. 2002, 119, 1420–1425. [Google Scholar] [CrossRef]
- Atkins, D.; Fink, K.; Slutsky, J. Better information for better health care: The Evidence-based Practice Center program and the Agency for Healthcare Research and Quality. Ann. Intern. Med. 2005, 142, 1035–1041. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef]
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels Containing Antibiofilm and Antimicrobial Agents Beneficial for Biofilm-Associated Wound Infection: Formulation Characterizations and In Vitro Study. AAPS PharmSciTech 2018, 19, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.R. Physical Properties of Glycerine. In Glycerin: A Key Cosmetic Ingredient; Jungermann, E., Sonntag, N.O.V., Eds.; CRC Press: New York, NY, USA, 2018; Volume 1, pp. 113–156. ISBN 9780203753071. [Google Scholar]
- Péterszegi, G.; Isnard, N.; Robert, A.M.; Robert, L. Studies on skin aging. Preparation and properties of fucose-rich oligo- and polysaccharides. Effect on fibroblast proliferation and survival. Biomed. Pharmacother. 2003, 57, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.W. The Dysvascular Foot: A System for Diagnosis and Treatment. Foot Ankle Int. 1981, 2, 64–122. [Google Scholar] [CrossRef]
- National Pressure Ulcer Advisory Panel; European Pressure Ulcer Advisory Panel; Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide, 2014th ed.; Haesler, E., Ed.; Cambridge Media: Osborne Park, Australia, 2014; ISBN 978-0-9579343-6-8. [Google Scholar]
- Chaby, G.; Viseux, V.; Ramelet, A.A.; Ganry, O.; Billet, A.; Lok, C. Refractory venous leg ulcers: A study of risk factors. Dermatol. Surg. 2006, 32, 512–519. [Google Scholar] [CrossRef]
- Margolis, D.J.; Allen-Taylor, L.; Hoffstad, O.; Berlin, J.A. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004, 12, 163–168. [Google Scholar] [CrossRef]




| EHO-85 (n = 35) | VariHesive (n = 34) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean/No. | SD/% | Mean/No. | SD/% | p | ||
| Women | 23 | 65.7% | 25 | 73.5% | |||
| Age (years) | 74.06 | 14.86 | 79.03 | 14.80 | |||
| BMI (kg/m2) | 27.04 | 6.85 | 29.61 | 9.91 | |||
| Diabetes mellitus | 10 | 28.6% | 9 | 26.5% | |||
| Current smoker | 4 | 11.4% | 2 | 5.9% | |||
| Alcohol (yes) | 5 | 14.3% | 1 | 2.9% | |||
| Patient status | 0.561 | ||||||
| Health-center care | 16 | 23.2% | 10 | 28.6% | 6 | 17.6% | |
| Home care | 34 | 49.3% | 16 | 45.7% | 18 | 52.9% | |
| Residential care (institutionalized) | 19 | 27.5% | 9 | 25.7% | 10 | 29.4% | |
| Degree of autonomy | 0.389 | ||||||
| Walks easily | 14 | 20.3% | 9 | 25.7% | 5 | 14.7% | |
| Walks with difficulty | 22 | 31.9% | 9 | 25.7% | 13 | 38.2% | |
| Confined to bed | 33 | 47.8% | 17 | 48.6% | 16 | 47.1% | |
| Lower-limb mobility | 0.274 | ||||||
| Full mobility | 17 | 24.6% | 10 | 28.6% | 7 | 20.6% | |
| Reduced mobility | 24 | 34.8% | 9 | 25.7% | 15 | 44.1% | |
| Immobility | 28 | 40.6% | 16 | 45.7% | 12 | 35.3% | |
| Blood test | |||||||
| Serum albumin (3.40–5.00 g/dL) | 3.61 | 0.48 | 3.57 | 0.51 | 0.665 | ||
| Creatinine clearance (80–120 mL/min) | 106.1 | 44.1 | 111.4 | 47.7 | 0.43 | ||
| EHO-85 (n = 35) | VariHesive (n = 34) | |||
|---|---|---|---|---|
| Ulcer | Mean/No. | SD/% | Mean/No. | SD/% |
| Etiology | ||||
| Venous | 14 | 40.0% | 14 | 41.2% |
| Ankle brachial index | 0.99 | 0.09 | 1.04 | 0.18 |
| Pressure | 20 | 57.1% | 19 | 55.9% |
| EPUAP II | 7 | 35.0% | 7 | 36.8% |
| EPUAP III | 13 | 65.0% | 12 | 63.2% |
| Diabetic foot | 1 | 2.9% | 1 | 2.9% |
| Wagner I | 1 | 100.0% | 0 | 0.0% |
| Wagner II | 0 | 0.0% | 1 | 100.0% |
| Ulcers/patient | ||||
| 1 | 21 | 60.0% | 18 | 52.9% |
| 2 | 8 | 22.9% | 10 | 29.4% |
| ≥3 | 6 | 17.1% | 6 | 17.6% |
| Evolution time (months) | 15.74 | 9.72 | 17.75 | 9.46 |
| Wound area (cm2) | 6.67 | 11.04 | 4.65 | 4.75 |
| Wound area | ||||
| ≤10 cm2 | 23 | 65.7% | 23 | 67.6% |
| >10 cm2 | 12 | 34.3% | 11 | 32.4% |
|
Granulation tissue (% over total ulcer) | 70.57 | 41.40 | 83.13 | 31.79 |
| Exudate | ||||
| None | 5 | 14.3% | 4 | 11.8% |
| Low | 17 | 48.6% | 13 | 38.2% |
| Intermediate | 10 | 28.5% | 14 | 41.2% |
| High | 3 | 8.6% | 3 | 8.8% |
| Recurrent ulcer | 20 | 57.1% | 16 | 47.1% |
| Previous hospitalization(s) due to treated ulcer(s) | 8 | 22.9% | 2 | 5.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdú-Soriano, J.; Casado-Díaz, A.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Moreno-Moreno, P.; Dorado, G.; Quesada-Gómez, J.M.; Rodríguez-Mañas, L.; Lázaro-Martínez, J.L. Hard-to-Heal Wound Healing: Superiority of Hydrogel EHO-85 (Containing Olea europaea Leaf Extract) vs. a Standard Hydrogel. A Randomized Controlled Trial. Gels 2023, 9, 962. https://doi.org/10.3390/gels9120962
Verdú-Soriano J, Casado-Díaz A, de Cristino-Espinar M, Luna-Morales S, Dios-Guerra C, Moreno-Moreno P, Dorado G, Quesada-Gómez JM, Rodríguez-Mañas L, Lázaro-Martínez JL. Hard-to-Heal Wound Healing: Superiority of Hydrogel EHO-85 (Containing Olea europaea Leaf Extract) vs. a Standard Hydrogel. A Randomized Controlled Trial. Gels. 2023; 9(12):962. https://doi.org/10.3390/gels9120962
Chicago/Turabian StyleVerdú-Soriano, José, Antonio Casado-Díaz, Marisol de Cristino-Espinar, Silvia Luna-Morales, Caridad Dios-Guerra, Paloma Moreno-Moreno, Gabriel Dorado, José Manuel Quesada-Gómez, Leocadio Rodríguez-Mañas, and José Luis Lázaro-Martínez. 2023. "Hard-to-Heal Wound Healing: Superiority of Hydrogel EHO-85 (Containing Olea europaea Leaf Extract) vs. a Standard Hydrogel. A Randomized Controlled Trial" Gels 9, no. 12: 962. https://doi.org/10.3390/gels9120962
APA StyleVerdú-Soriano, J., Casado-Díaz, A., de Cristino-Espinar, M., Luna-Morales, S., Dios-Guerra, C., Moreno-Moreno, P., Dorado, G., Quesada-Gómez, J. M., Rodríguez-Mañas, L., & Lázaro-Martínez, J. L. (2023). Hard-to-Heal Wound Healing: Superiority of Hydrogel EHO-85 (Containing Olea europaea Leaf Extract) vs. a Standard Hydrogel. A Randomized Controlled Trial. Gels, 9(12), 962. https://doi.org/10.3390/gels9120962