Thermoresponsive Alginate-Graft-pNIPAM/Methyl Cellulose 3D-Printed Scaffolds Promote Osteogenesis In Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Alg-g-PNIPAM
2.2. Thermosensitivity Measurements and Rheological Evaluation
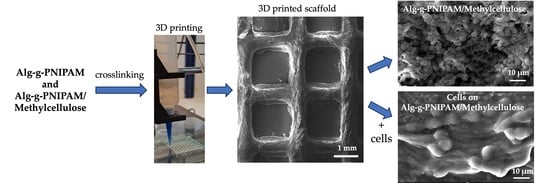
2.3. Characterization and Erosion Studies of 3D-Printed Scaffolds
2.3.1. Structural Characterization
2.3.2. Thermal Characterization
2.3.3. Morphological Characterization
2.3.4. Erosion Studies
2.4. Evaluation of Cytocompatibility, Cell Adhesion, Viability and Proliferation
2.4.1. Cell Viability and Proliferation
2.4.2. Cell Adhesion and Morphology Evaluation
2.4.3. Evaluation of Osteogenic Differentiation Markers ALP Activity and Calcium and Collagen Production by Cells Cultured on Alg-g-PNIPAM and Alg-g-PNIPAM/MC Scaffolds
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Amino-Terminated PNIPAM-NH2 Side
4.3. Synthesis of the Alg-g-PNIPAM
4.4. Proton Nuclear Magnetic Resonance (1H NMR)
4.5. Hydrogel Preparation
4.6. Rheological Studies
4.7. Determination of the LCST
4.8. 3D Scaffold Design and Manufacturing
4.9. Structural Characterization
4.10. Thermal Characterization
4.11. Morphological Characterization
4.12. Cell Culture Maintenance
4.13. Cell Viability and Proliferation Assessment
4.14. Cell Adhesion and Morphology
4.15. Osteogenic Potential Evaluation of Pre-Osteoblasts Seeded onto Scaffolds by Determination of the ALP Activity, Collagen and Calcium Secretion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Douroumis, D.; Boateng, J. 3D Printed Scaffolds for Wound Healing and Tissue Regeneration. In Therapeutic Dressings and Wound Healing Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 385–398. [Google Scholar] [CrossRef]
- Kontogianni, G.-I.; Bonatti, A.F.; De Maria, C.; Naseem, R.; Melo, P.; Coelho, C.; Vozzi, G.; Dalgarno, K.; Quadros, P.; Vitale-Brovarone, C.; et al. Promotion of In Vitro Osteogenic Activity by Melt Extrusion-Based PLLA/PCL/PHBV Scaffolds Enriched with Nano-Hydroxyapatite and Strontium Substituted Nano-Hydroxyapatite. Polymers 2023, 15, 1052. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- Singh, R.; Deshmukh, S.A.; Kamath, G.; Sankaranarayanan, S.K.R.S.; Balasubramanian, G. Controlling the aqueous solubility of PNIPAM with hydrophobic molecular units. Comput. Mater. Sci. 2017, 126, 191–203. [Google Scholar] [CrossRef]
- Lencina, S.; Iatridi, Z.; Villar, M.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wang, X.; Pi, M.; Yan, B.; Ran, R. A rapidly responsive, controllable, and reversible photo-thermal dual response hydrogel. Polymer 2021, 237, 124344. [Google Scholar] [CrossRef]
- Zhang, J.; Yun, S.; Karami, A.; Jing, B.; Zannettino, A.; Du, Y.; Zhang, H. 3D printing of a thermosensitive hydrogel for skin tissue engineering: A proof of concept study. Bioprinting 2020, 19, e00089. [Google Scholar] [CrossRef]
- Fitzsimmons, R.; Aquilino, M.; Quigley, J.; Chebotarev, O.; Tarlan, F.; Simmons, C. Generating vascular channels within hydrogel constructs using an economical open-source 3D bioprinter and thermoreversible gels. Bioprinting 2018, 9, 7–18. [Google Scholar] [CrossRef]
- Celikkin, N.; Simó Padial, J.; Costantini, M.; Hendrikse, H.; Cohn, R.; Wilson, C.; Rowan, A.; Święszkowski, W. 3D Printing of Thermoresponsive Polyisocyanide (PIC) Hydrogels as Bioink and Fugitive Material for Tissue Engineering. Polymers 2018, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ahmad, T.; Haider, M.S.; Hahn, L.; Stahlhut, P.; Groll, J.; Luxenhofer, R. A thermogelling organic-inorganic hybrid hydrogel with excellent printability, shape fidelity and cytocompatibility for 3D bioprinting. Biofabrication 2022, 14, 025005. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Mahboubian, A.; Vllasaliu, D.; Dorkoosh, F.A.; Stolnik, S. Temperature-Responsive Methylcellulose–Hyaluronic Hydrogel as a 3D Cell Culture Matrix. Biomacromolecules 2020, 21, 4737–4746. [Google Scholar] [CrossRef] [PubMed]
- Contessi Negrini, N.; Bonetti, L.; Contili, L.; Farè, S. 3D printing of methylcellulose-based hydrogels. Bioprinting 2018, 10, e00024. [Google Scholar] [CrossRef]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it up: The promise of stimuli-responsive polymer systems in biomedical science. Chem. Rec. 2023, e202300217. [Google Scholar] [CrossRef]
- Guo, H.; de Magalhaes Goncalves, M.; Ducouret, G.; Hourdet, D. Cold and Hot Gelling of Alginate-graft-PNIPAM: A Schizophrenic Behavior Induced by Potassium Salts. Biomacromolecules 2018, 19, 576–587. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Soares, J.; Santos, J.; Chierice, G.; Cavalheiro, É. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Toto, E.; Santonicola, M.G. Conductive and Thermo-Responsive Composite Hydrogels with Poly(N-isopropylacrylamide) and Carbon Nanotubes Fabricated by Two-Step Photopolymerization. Polymers 2023, 15, 1022. [Google Scholar] [CrossRef]
- Shekhar, S.; Mukherjee, M.; Sen, A. Studies on thermal and swelling properties of Poly (NIPAM-co-2-HEA) based hydrogels. Adv. Mater. Res. 2012, 1, 269. [Google Scholar] [CrossRef]
- Saeed, A.; Georget, D.M.R.; Mayes, A.G. Solid-state thermal stability and degradation of a family of poly(N-isopropylacrylamide-co-hydroxymethylacrylamide) copolymers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5848–5855. [Google Scholar] [CrossRef]
- Liu, S.; Kilian, D.; Ahlfeld, T.; Hu, Q.; Gelinsky, M. Egg white improves the biological properties of an alginate-methylcellulose bioink for 3D bioprinting of volumetric bone constructs. Biofabrication 2023, 15, 025013. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 2003, 24, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Bousnaki, M.; Bakopoulou, A.; Papadogianni, D.; Barkoula, N.-M.; Alpantaki, K.; Kritis, A.; Chatzinikolaidou, M.; Koidis, P. Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 97. [Google Scholar] [CrossRef]
- Kim, H.; Witt, H.; Oswald, T.A.; Tarantola, M. Adhesion of Epithelial Cells to PNIPAm Treated Surfaces for Temperature-Controlled Cell-Sheet Harvesting. ACS Appl. Mater. Interfaces 2020, 12, 33516–33529. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, Y.; Ma, S.; Duan, S.; Yang, X.; Gao, P.; Zhang, X.; Cai, Q. Effective Bone Regeneration Using Thermosensitive Poly(N-Isopropylacrylamide) Grafted Gelatin as Injectable Carrier for Bone Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 19006–19015. [Google Scholar] [CrossRef]
- Fermani, M.; Platania, V.; Kavasi, R.-M.; Karavasili, C.; Zgouro, P.; Fatouros, D.; Chatzinikolaidou, M.; Bouropoulos, N. 3D-Printed Scaffolds from Alginate/Methyl Cellulose/Trimethyl Chitosan/Silicate Glasses for Bone Tissue Engineering. Appl. Sci. 2021, 11, 8677. [Google Scholar] [CrossRef]
- von Strauwitz Né Ahlfeld, T.; Tiodorovic Neé Guduric, V.; Duin, S.; Akkineni, A.R.; Schütz, K.; Kilian, D.; Emmermacher, J.; Cubo Mateo, N.; Dani, S.; von Witzleben, M.; et al. Methylcellulose—A versatile printing material that enables biofabrication of tissue equivalents with high shape fidelity. Biomater. Sci. 2020, 8, 2102–2110. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, B.; Zhang, X.; Huang, S.; Wa, Q. 3D printed concentrated alginate/GelMA hollow-fibers-packed scaffolds with nano apatite coatings for bone tissue engineering. Int. J. Biol. Macromol. 2022, 202, 366–374. [Google Scholar] [CrossRef]
- Liao, H.-T.; Chen, C.-T.; Chen, J.-P. Osteogenic Differentiation and Ectopic Bone Formation of Canine Bone Marrow-Derived Mesenchymal Stem Cells in Injectable Thermo-Responsive Polymer Hydrogel. Tissue Eng. Part C Methods 2011, 17, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Saravanou, S.F.; Ioannidis, K.; Dimopoulos, A.; Paxinou, A.; Kounelaki, F.; Varsami, S.M.; Tsitsilianis, C.; Papantoniou, I.; Pasparakis, G. Dually crosslinked injectable alginate-based graft copolymer thermoresponsive hydrogels as 3D printing bioinks for cell spheroid growth and release. Carbohydr. Polym. 2023, 312, 120790. [Google Scholar] [CrossRef] [PubMed]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Alginate-g-PNIPAM-Based Thermo/Shear-Responsive Injectable Hydrogels: Tailoring the Rheological Properties by Adjusting the LCST of the Grafting Chains. Int. J. Mol. Sci. 2021, 22, 3824. [Google Scholar] [CrossRef] [PubMed]












| Grafting Chain | Mn (g/mol) a |
|---|---|
| PNIPAM-NH2 | 13,740 |
| Graft Copolymer | Mw (g/mol) a | % Molar Composition Alg/Grafting Chain (mol/mol) b | % Weight Composition Alg/Grafting Chains (w/w) | Number of PNIPAM Side Chains Per NaALG Backbone b |
|---|---|---|---|---|
| Alg-g-PNIPAM | 167,470 | 74.4/25.6 | 83.6/16.4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gialouri, A.; Saravanou, S.F.; Loukelis, K.; Chatzinikolaidou, M.; Pasparakis, G.; Bouropoulos, N. Thermoresponsive Alginate-Graft-pNIPAM/Methyl Cellulose 3D-Printed Scaffolds Promote Osteogenesis In Vitro. Gels 2023, 9, 984. https://doi.org/10.3390/gels9120984
Gialouri A, Saravanou SF, Loukelis K, Chatzinikolaidou M, Pasparakis G, Bouropoulos N. Thermoresponsive Alginate-Graft-pNIPAM/Methyl Cellulose 3D-Printed Scaffolds Promote Osteogenesis In Vitro. Gels. 2023; 9(12):984. https://doi.org/10.3390/gels9120984
Chicago/Turabian StyleGialouri, Aikaterini, Sofia Falia Saravanou, Konstantinos Loukelis, Maria Chatzinikolaidou, George Pasparakis, and Nikolaos Bouropoulos. 2023. "Thermoresponsive Alginate-Graft-pNIPAM/Methyl Cellulose 3D-Printed Scaffolds Promote Osteogenesis In Vitro" Gels 9, no. 12: 984. https://doi.org/10.3390/gels9120984
APA StyleGialouri, A., Saravanou, S. F., Loukelis, K., Chatzinikolaidou, M., Pasparakis, G., & Bouropoulos, N. (2023). Thermoresponsive Alginate-Graft-pNIPAM/Methyl Cellulose 3D-Printed Scaffolds Promote Osteogenesis In Vitro. Gels, 9(12), 984. https://doi.org/10.3390/gels9120984