Efficacy of 5% Aminolaevulinic Acid and Red Light on Enterococcus faecalis in Infected Root Canals
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
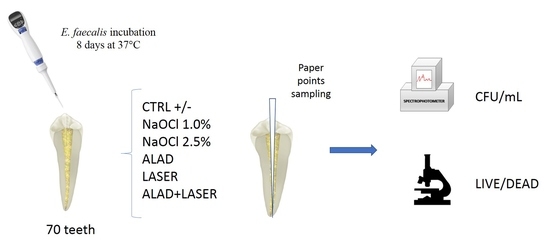
4. Materials and Methods
4.1. Sample Preparation
4.2. Strain Culture Condition
4.3. Aladent
4.4. Light Source and Fiber TIP
4.5. Study Model
- ALAD: samples incubated 45 min with ALAD gel;
- LASER: samples irradiated 7 min with the optical fiber at 50 mW;
- ALAD + LASER: samples incubated 45 min with ALAD and then irradiated 7 min with the optical fiber at 50 mW;
- NaOCl 2.5%: samples incubated 15 min with sodium hypochlorite 2.5%;
- NaOCl 1.0%: samples incubated 15 min with sodium hypochlorite 1%;
- CTRL: positive control, only Enterococcus faecalis ATCC 29212, received no treatment;
- Negative control: uninoculated sample.
4.6. Determination of Colony-Forming Units (CFU)
4.7. Cell Viability Assay and Fluorescent Microscopy
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, L.P.; Nunes, G.P.; Ferrisse, T.M.; Strazzi-Sahyon, H.B.; Cintra, L.T.; dos Santos, P.H.; Sivieri-Araujo, G. Antimicrobial photodynamic therapy in endodontic reintervention: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2022, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, D.; Toia, C.C.; Orozco, E.I.F.; Khoury, R.D.; Cardoso, F.G.D.R.; Alves, M.C.; Carvalho, C.A.T.; Valera, M.C. Effectiveness in the Removal of Endotoxins and Microbiological Profile in Primary Endodontic Infections Using 3 Different Instrumentation Systems: A Randomized Clinical Study. J. Endod. 2017, 43, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.C.; De Figueiredo, J.A.P.; Steier, L.; Weber, J.B.B. Photodynamic therapy in endodontics: A literature review. Photomed. Laser Surg. 2015, 33, 175–182. [Google Scholar] [CrossRef]
- Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Using ultraviolet (UV) light emitting diodes (LED) to create sterile root canals and to treat endodontic infections. Curr. Opin. Biomed. Eng. 2022, 23, 1–10. [Google Scholar]
- Strazzi-Sahyon, H.B.; Oliveira, A.K.L.; Carvalho, A.P.; Figueiredo, R.B.; Cintra, L.T.A.; Gomes-Filho, J.E.; dos Santos, P.H.; Sivieri-Araujo, G. Influence of photodynamic therapy and intracanal medication on Martens hardness, elastic modulus and bond strength of glass-fiber posts to endodontically treated root dentin. Photodiagnosis Photodyn. Ther. 2021, 36, 1–8. [Google Scholar] [CrossRef]
- Plotino, G.; Cortese, T.; Grande, N.M.; Leonardi, D.; DI Giorgio, G.; Testarelli, L.; Gambarini, G. New Technologies to Improve Root Canal Disinfection. Braz. Dent. J. 2016, 27, 3–8. [Google Scholar] [CrossRef]
- Nair, P.N.R.; Henry, S.; Cano, V.; Vera, J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 231–252. [Google Scholar] [CrossRef]
- Haapasalo, M.; Ørstavik, D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 1987, 66, 1375–1379. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhou, X.; Zeng, J.; Ren, Z.; Lei, L.; Kang, D.; Zhang, K.; Zou, J.; Li, Y. Inhibition of Enterococcus faecalis Growth and Biofilm Formation by Molecule Targeting Cyclic di-AMP Synthetase Activity. J. Endod. 2018, 44, 1381–1388. [Google Scholar] [CrossRef]
- Saatchi, M.; Shokraneh, A.; Navaei, H.; Maracy, M.R.; Shojaei, H. Antibacterial effect of calcium hydroxide combined with chlorhexidine on Enterococcus faecalis: A systematic review and meta-analysis. J. Appl. Oral Sci. 2014, 37, 356–365. [Google Scholar] [CrossRef]
- Zancan, R.F.; Calefi, P.H.S.; Borges, M.M.B.; Lopes, M.R.M.; de Andrade, F.B.; Vivan, R.R.; Duarte, M.A.H. Antimicrobial activity of intracanal medications against both Enterococcus faecalis and Candida albicans biofilm. Microsc. Res. Tech. 2019, 82, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-W.; Lee, Y.-L.; Hsiao, S.-H.; Lin, H.-P. Bacteria in the apical root canals of teeth with apical periodontitis. J. Formos. Med Assoc. 2017, 116, 448–456. [Google Scholar] [CrossRef]
- Petrini, M.; Trentini, P.; Tripodi, D.; Spoto, G.; D’Ercole, S. In vitro antimicrobial activity of LED irradiation on Pseudomonas aeruginosa. J. Photochem. Photobiol. B Biol. 2017, 168, 25–29. [Google Scholar] [CrossRef]
- Petrini, M.; Spoto, G.; Scarano, A.; D’Arcangelo, C.; Tripodi, D.; Di Fermo, P.; D’Ercole, S. Near-infrared LEDS provide persistent and increasing protection against E. faecalis. J. Photochem. Photobiol. B Biol. 2019, 197, 111527. [Google Scholar] [CrossRef]
- D’'Ercole, S.; Di Fermo, P.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Scarano, A.; Tripodi, D.; Cellini, L.; Petrini, M. Near-infrared NIR irradiation and sodium hypochlorite: An efficacious association to counteract the Enterococcus faecalis biofilm in endodontic infections. J. Photochem. Photobiol. B Biol. 2020, 210, 111989. [Google Scholar] [CrossRef]
- Soares, L.G.; Crugeira, P.J.; Nunes, I.P.; Santos, A.S.; Cangussú, M.C.; de Almeida, P.F.; Pinheiro, A.L.; Habib, F.A. Oral microbiological control by photodynamic action in orthodontic patients. Photodiagnosis Photodyn. Ther. 2019, 28, 221–225. [Google Scholar] [CrossRef]
- Strazzi Sahyon, H.B.; Pereira da Silva, P.; Silva de Oliveira, M.; Angelo Cintra, L.T.; Gomes-Filho, J.E.; Henrique Dos Santos, P.; Sivieri-Araujo, G. Effect of photodynamic therapy on the mechanical properties and bond strength of glass-fiber posts to endodontically treated intraradicular dentin. J. Prosthet. Dent. 2018, 120, 317.e1–317.e7. [Google Scholar] [CrossRef]
- D’Ercole, S.; Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Tripodi, D.; Iezzi, G.; Piattelli, A.; Petrini, M. 5-Aminolevulinic Acid and Red Led in Endodontics: A Narrative Review and Case Report. Gels 2022, 8, 697. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a novel gel containing 5-aminolevulinic acid and red LED against bacteria involved in peri-implantitis and other oral infections. J. Photochem. Photobiol. B Biol. 2020, 205, 111826. [Google Scholar] [CrossRef]
- Yang, X.; Palasuberniam, P.; Kraus, D.; Chen, B. Aminolevulinic Acid-Based Tumor Detection and Therapy: Molecular Mechanisms and Strategies for Enhancement. Int. J. Mol. Sci. 2015, 16, 25865–25880. [Google Scholar] [CrossRef]
- Petrini, M.; Pierfelice, T.V.; D’Amico, E.; Carlesi, T.; Iezzi, G.; D’Arcangelo, C.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Comparison between Single and Multi-LED Emitters for Photodynamic Therapy: An In Vitro Study on Enterococcus faecalis and Human Gingival Fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 3048. [Google Scholar] [CrossRef] [PubMed]
- Blanken, J.W.; Verdaasdonk, R.M. Laser treatment in root canals. Effective by explosive vapour bubbles. Ned. Tijdschr. Tandheelkd. 2009, 116, 355–360. [Google Scholar] [PubMed]
- Ng, R.; Singh, F.; Papamanou, D.A.; Song, X.; Patel, C.; Holewa, C.; Patel, N.; Klepac-Ceraj, V.; Fontana, C.R.; Kent, R.; et al. Endodontic Photodynamic Therapy Ex Vivo. J. Endod. 2011, 37, 217–222. [Google Scholar] [CrossRef]
- Teixeira, P.A.; Coelho, M.S.; Kato, A.S.; Fontana, C.E.; Bueno, C.E.; Pedro-Rocha, D.G. Cytotoxicity assessment of 1% peracetic acid, 2.5% sodium hypochlorite and 17% EDTA on FG11 and FG15 human fibroblasts. Acta Odontol. Latinoam. 2018, 31, 11–15. [Google Scholar]
- Simbula, G.; Dettori, C.; Camboni, T.; Cotti, E. Comparison of Tetraacetylethylendiamine + Sodium Perborate and Sodium Hypochlorite Cytotoxicity on L929 Fibroblasts. J. Endod. 2010, 36, 1516–1520. [Google Scholar] [CrossRef]
- Garcez, A.S.; Arantes-Neto, J.G.; Sellera, D.P.; Fregnani, E.R. Effects of antimicrobial photodynamic therapy and surgical endodontic treatment on the bacterial load reduction and periapical lesion healing. Three years follow up. Photodiagnosis Photodyn. Ther. 2015, 12, 575–580. [Google Scholar] [CrossRef]
- Zorita-García, M.; Alonso-Ezpeleta, L.Ó.; Cobo, M.; Del Campo, R.; Rico-Romano, C.; Mena-Álvarez, J.; Zubizarreta-Macho, Á. Photodynamic therapy in endodontic root canal treatment significantly increases bacterial clearance, preventing apical periodontitis. Quintessence Int. 2019, 50, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Ghorbanzadeh, R.; Parker, S.; Chiniforush, N.; Bahador, A. The evaluation of cultivable microbiota profile in patients with secondary endodontic infection before and after photo-activated disinfection. Photodiagnosis Photodyn. Ther. 2017, 18, 198–203. [Google Scholar] [CrossRef]
- Rabello, D.; Corazza, B.J.; Ferreira, L.L.; Santamaria, M.; Gomes, A.P.M.; Martinho, F. Does supplemental photodynamic therapy optimize the disinfection of bacteria and endotoxins in one-visit and two-visit root canal therapy? A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2017, 19, 205–211. [Google Scholar] [CrossRef]
- Asnaashari, M.; Homayuni, H.; Paymanpour, P. The Antibacterial Effect of Additional Photodynamic Therapy in Failed Endodontically Treated Teeth: A Pilot Study. J. Lasers Med. Sci. 2016, 7, 238–242. [Google Scholar] [CrossRef]
- Tennert, C.; Drews, A.; Walther, V.; Altenburger, M.; Karygianni, L.; Wrbas, K.; Hellwig, E.; Al-Ahmad, A. Ultrasonic activation and chemical modification of photosensitizers enhances the effects of photodynamic therapy against Enterococcus faecalis root-canal isolates. Photodiagnosis Photodyn. Ther. 2015, 12, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prashant, J.; Ginimuge, R.; Jyothi, S.D. Methylene Blue: Revisited. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar]
- Di Lodovico, S.; Diban, F.; Di Fermo, P.; Petrini, M.; Fontana, A.; Di Giulio, M.; Piattelli, A.; D’Ercole, S.; Cellini, L. Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management. Int. J. Mol. Sci. 2022, 23, 6942. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlesi, T.; Dotta, T.C.; Pierfelice, T.V.; D’Amico, E.; Lepore, S.; Tripodi, D.; Piattelli, A.; D’Ercole, S.; Petrini, M. Efficacy of 5% Aminolaevulinic Acid and Red Light on Enterococcus faecalis in Infected Root Canals. Gels 2023, 9, 125. https://doi.org/10.3390/gels9020125
Carlesi T, Dotta TC, Pierfelice TV, D’Amico E, Lepore S, Tripodi D, Piattelli A, D’Ercole S, Petrini M. Efficacy of 5% Aminolaevulinic Acid and Red Light on Enterococcus faecalis in Infected Root Canals. Gels. 2023; 9(2):125. https://doi.org/10.3390/gels9020125
Chicago/Turabian StyleCarlesi, Teocrito, Tatiane Cristina Dotta, Tania Vanessa Pierfelice, Emira D’Amico, Stefania Lepore, Domenico Tripodi, Adriano Piattelli, Simonetta D’Ercole, and Morena Petrini. 2023. "Efficacy of 5% Aminolaevulinic Acid and Red Light on Enterococcus faecalis in Infected Root Canals" Gels 9, no. 2: 125. https://doi.org/10.3390/gels9020125
APA StyleCarlesi, T., Dotta, T. C., Pierfelice, T. V., D’Amico, E., Lepore, S., Tripodi, D., Piattelli, A., D’Ercole, S., & Petrini, M. (2023). Efficacy of 5% Aminolaevulinic Acid and Red Light on Enterococcus faecalis in Infected Root Canals. Gels, 9(2), 125. https://doi.org/10.3390/gels9020125