Study on the Interaction of Plasma-Polymerized Hydrogel Coatings with Aqueous Solutions of Different pH
Abstract
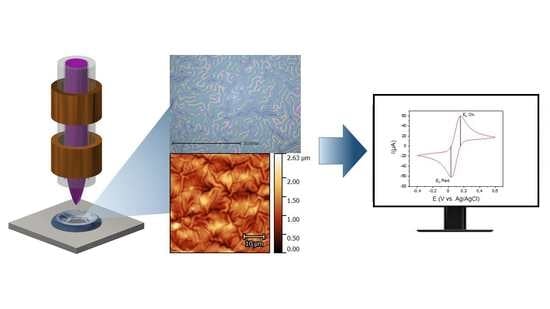
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Hydrogel Coating
2.2. Surface Topography
2.3. Electrochemical Characterization
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Plasma Source and Plasma Polymerization
4.3. Surface Characterization
4.4. Electrochemical Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.F.; Kumpgdee-Vollrath, M.; Franco, M.K.K.D.; Yokaichiya, F.; de Araujo, D.R. Supramolecular structure organization and rheological properties modulate the performance of hyaluronic acid-loaded thermosensitive hydrogels as drug-delivery systems. J. Colloid Interf. Sci. 2023, 630, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Xu, F.; Wei, W.; Yang, C.; Wang, D.; Shi, X. Electrochemical synthesis of chitosan/silver nanoparticles multilayer hydrogel coating with pH-dependent controlled release capability and antibacterial property. Colloids Surf. B Biointerfaces 2021, 202, 111711. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [Green Version]
- Volpi, M.; Paradiso, A.; Costantini, M.; Swieszkowski, W. Hydrogel-based fiber biofabrication techniques for skeletal muscle tissue engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef]
- Rahman, M.S.A.; Mukhopadhyay, S.C.; Yu, P.-L. Coating and Immobilization of Sensors. In Novel Sensors for Food Inspection: Modelling, Fabrication and Experimentation; Springer: Berlin/Heidelberg, Germany, 2014; pp. 73–85. [Google Scholar]
- Zhang, X.; Kwon, K.; Henriksson, J.; Luo, J.; Wu, M.C. A large-scale microelectromechanical-systems-based silicon photonics LiDAR. Nature 2022, 603, 253–258. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Zhang, N.; Li, J.; Zhou, P.; Hu, F.; Rong, Y.; Lu, B.; Gu, G. High-stretchability, ultralow-hysteresis conducting polymer hydrogel strain sensors for soft machines. Adv. Mater. 2022, 34, e2203650. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Yan, L.W.; Wang, M.H.; Wang, K.F.; Fang, L.M.; Zhou, J.; Fang, J.; Ren, F.Z.; Lu, X. Transparent, adhesive, and conductive hydrogel for soft bioelectronics based on light-transmitting polydopamine-doped polypyrrole nanofibrils. Chem. Mater. 2018, 30, 5561–5572. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.; et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68. [Google Scholar] [CrossRef]
- Tian, F.; Yu, J.; Wang, W.; Zhao, D.; Cao, J.; Zhao, Q.; Wang, F.; Yang, H.; Wu, Z.; Xu, J.; et al. Design of adhesive conducting PEDOT-MeOH:PSS/PDA neural interface via electropolymerization for ultrasmall implantable neural microelectrodes. J. Colloid Interface Sci. 2023, 638, 339–348. [Google Scholar] [CrossRef]
- Young, A.T.; Cornwell, N.; Daniele, M.A. Neuro-nano interfaces: Utilizing nano-coatings and nanoparticles to enable next-generation electrophysiological recording, neural stimulation, and biochemical modulation. Adv. Funct. Mater. 2018, 28, 1700239. [Google Scholar] [CrossRef]
- Thakar, H.; Sebastian, S.M.; Mandal, S.; Pople, A.; Agarwal, G.; Srivastava, A. Biomolecule-conjugated macroporous hydrogels for biomedical applications. ACS Biomater. Sci. Eng. 2019, 5, 6320–6341. [Google Scholar] [CrossRef]
- George, S.M.; Tandon, S.; Kandasubramanian, B. Advancements in hydrogel-functionalized immunosensing platforms. ACS Omega 2020, 5, 2060–2068. [Google Scholar] [CrossRef]
- Refojo, M.F.; Leong, F.L. Poly(methyl acrylate-co-hydroxyethyl acrylate) hydrogel implant material of strength and softness. J. Biomed. Mater. Res. 1981, 15, 497–509. [Google Scholar] [CrossRef]
- Kou, J.H.; Fleisher, D.; Amidon, G.L. Modeling drug release from dynamically swelling poly(hydroxyethyl methacrylate-Co-methacrylic acid) hydrogels. J. Control. Release 1990, 12, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Lesho, M.J.; Sheppard, N.F., Jr. A method for studying swelling kinetics based on measurement of electrical conductivity. Polym. Gels Netw. 1998, 5, 503–523. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Lan, T.; Carroll, D.R.; Georgiou, T.K. Tricomponent thermoresponsive polymers based on an amine-containing monomer with tuneable hydrophobicity: Effect of composition. Eur. Polym. J. 2020, 130, 109655. [Google Scholar] [CrossRef]
- Monteiro, G.A.A.; de Sousa, R.G.; da Silva, W.M.; Gastelois, P.L.; Macedo, W.A.d.A.; de Sousa, E.M.B. Microwave radiation-assisted covalent functionalization of boron nitride nanotubes and their grafting with cationic thermo and pH-sensitive hydrogel. Appl. NanoSci. 2021, 11, 505–520. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Meng, Z.; Xie, B.; Yu, X.; Su, G.; Wang, L. Epoxy coating with excellent anticorrosion and pH-responsive performances based on DEAEMA modified mesoporous silica nanomaterials. Colloids Surf. A Phys. Eng. Asp. 2022, 634, 127951. [Google Scholar] [CrossRef]
- Xu, F.J.; Neoh, K.G.; Kang, E.T. Bioactive surfaces and biomaterials via atom transfer radical polymerization. Prog. Polym. Sci. 2009, 34, 719–761. [Google Scholar] [CrossRef]
- Willner, I. Stimuli-controlled hydrogels and their applications. Acc. Chem. Res. 2017, 50, 657–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkhoury, K.; Russell, C.S.; Sanchez-Gonzalez, L.; Mostafavi, A.; Williams, T.J.; Kahn, C.; Peppas, N.A.; Arab-Tehrany, E.; Tamayol, A. Soft-nanoparticle functionalization of natural hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2019, 8, e1900506. [Google Scholar] [CrossRef] [PubMed]
- Koc, J.; Schonemann, E.; Amuthalingam, A.; Clarke, J.; Finlay, J.A.; Clare, A.S.; Laschewsky, A.; Rosenhahn, A. Low-fouling thin hydrogel coatings made of photo-cross-linked polyzwitterions. Langmuir 2019, 35, 1552–1562. [Google Scholar] [CrossRef]
- Levien, M.; Fricke, K. Fabrication of hydrogel coatings by atmospheric-pressure plasma polymerization Function by structure and chemistry. Mater. Today 2020, 41, 316–317. [Google Scholar] [CrossRef]
- Ma, C.L.; Wang, L.; Nikiforov, A.; Onyshchenko, Y.; Cools, P.; Ostrikov, K.; De Geyter, N.; Morent, R. Atmospheric-pressure plasma assisted engineering of polymer surfaces: From high hydrophobicity to superhydrophilicity. Appl. Surf. Sci. 2021, 535, 147032. [Google Scholar] [CrossRef]
- Makhneva, E.; Barillas, L.; Farka, Z.; Pastucha, M.; Skladal, P.; Weltmann, K.D.; Fricke, K. Functional Plasma Polymerized Surfaces for Biosensing. ACS Appl. Mater. Interfaces 2020, 12, 17100–17112. [Google Scholar] [CrossRef]
- Kálal, J. The use of methacrylic polymers in medicine. Macromol. Chem. Phys. 1984, 7, 31–39. [Google Scholar] [CrossRef]
- Chung, J.T.; Vlugt-Wensink, K.D.; Hennink, W.E.; Zhang, Z. Effect of polymerization conditions on the network properties of dex-HEMA microspheres and macro-hydrogels. Int. J. Pharm. 2005, 288, 51–61. [Google Scholar] [CrossRef]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource; ACS Publications: Washington, DC, USA, 2010. [Google Scholar]
- Gu, J.; Li, X.; Ma, H.; Guan, Y.; Zhang, Y. One-step synthesis of PHEMA hydrogel films capable of generating highly ordered wrinkling patterns. Polymer 2017, 110, 114–123. [Google Scholar] [CrossRef]
- Zhang, J.; Peppas, N.A. Synthesis and characterization of pH-and temperature-sensitive poly (methacrylic acid)/poly (N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules 2000, 33, 102–107. [Google Scholar] [CrossRef]
- Parlak, O.; Ashaduzzaman, M.; Kollipara, S.B.; Tiwari, A.; Turner, A.P. Switchable bioelectrocatalysis controlled by dual stimuli-responsive polymeric interface. ACS Appl. Mater. Interfaces 2015, 7, 23837–23847. [Google Scholar] [CrossRef]
- Satish, C.S.; Shivakumar, H.G. Formulation and evaluation of self-regulated insulin delivery system based on poly(HEMA-co-DMAEMA) hydrogels. J. Macromol. Sci. A 2007, 44, 379–387. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sagredo-Oyarce, D.H.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Fabrication of micro and sub-micrometer wrinkled hydrogel surfaces through thermal and photocrosslinking processes. Polymer 2016, 101, 24–33. [Google Scholar] [CrossRef]
- Yongabi, D.; Khorshid, M.; Gennaro, A.; Jooken, S.; Duwe, S.; Deschaume, O.; Losada-Perez, P.; Dedecker, P.; Bartic, C.; Wubbenhorst, M.; et al. QCM-D study of time-resolved cell adhesion and detachment: Effect of surface free energy on eukaryotes and prokaryotes. ACS Appl. Mater. Interfaces 2020, 12, 18258–18272. [Google Scholar] [CrossRef]
- Rojewska, M.; Bartkowiak, A.; Strzemiecka, B.; Jamrozik, A.; Voelkel, A.; Prochaska, K. Surface properties and surface free energy of cellulosic etc mucoadhesive polymers. Carbohydr. Polym. 2017, 171, 152–162. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Ho, C.P.; Yasuda, H. Coatings and Surface Modification by Methane Plasma Polymerization. J. Appl. Polym. Sci. 1990, 39, 1541–1552. [Google Scholar] [CrossRef]
- Cook, A.D.; Sagers, R.D.; Pitt, W.G. Bacterial adhesion to poly(HEMA)-based hydrogels. J. Biomed. Mater. Res. 1993, 27, 119–126. [Google Scholar] [CrossRef]
- Klages, C.-P.; Höpfner, K.; Kläke, N.; Thyen, R. Surface functionalization at atmospheric pressure by DBD-based pulsed plasma polymerization. Plasma. Process. Polym. 2000, 5, 79–89. [Google Scholar] [CrossRef]
- Wang, Y.; Kozlovskaya, V.; Arcibal, I.G.; Cropek, D.M.; Kharlampiev, E. Highly swellable ultrathin poly(4-vinylpyridine) multilayer hydrogels with pH-triggered surface wettability. Soft Matter 2013, 9, 9420–9429. [Google Scholar] [CrossRef]
- Lin, W.; Nie, S.; Xiong, D.; Guo, X.; Wang, J.; Zhang, L. pH-responsive micelles based on (PCL)2(PDEA-b-PPEGMA)2 miktoarm polymer: Controlled synthesis, characterization, and application as anticancer drug carrier. Nanoscale Res. Lett. 2014, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besli, N.S.O.; Orakdogen, N. Thermomechanical analysis and pH-triggered elastic response of charge-balanced sulfonated poly (tertiary amine-methacrylate)-based terpolymer cryogels. Polymer 2020, 208, 122941. [Google Scholar] [CrossRef]
- Britten, C.N.; Lason, K.; Walters, K.B. Facile synthesis of tertiary amine pendant polymers by Cu0-mediated ATRP under aqueous conditions. Macromolecules 2021, 54, 10360–10369. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Alfaro-Cerda, P.A.; Veliz-Silva, D.F.; Sarabia-Vallejos, M.A.; Terraza, C.A.; Rodriguez-Hernandez, J. Micro-wrinkled hydrogel patterned surfaces using pH-sensitive monomers. App. Surf. Sci. 2018, 457, 902–913. [Google Scholar] [CrossRef]
- Stratakis, E.; Mateescu, A.; Barberoglou, M.; Vamvakaki, M.; Fotakis, C.; Anastasiadis, S.H. From superhydrophobicity and water repellency to superhydrophilicity: Smart polymer-functionalized surfaces. Chem. Commun. 2010, 46, 4136–4138. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, J. Wrinkled interfaces: Taking advantage of surface instabilities to pattern polymer surfaces. Prog. Polym. Sci. 2015, 42, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, J.B.; Moatsou, D.; Surapaneni, V.A.; Thielen, M.; Speck, T.; Wilts, B.D.; Steiner, U. Polymerization-Induced Wrinkled Surfaces with Controlled Topography as Slippery Surfaces for Colorado Potato Beetles. Adv. Mater. Interfaces 2020, 7, 2000129. [Google Scholar] [CrossRef]
- Ferreira, L.; Vidal, M.M.; Gil, M.H. Evaluation of poly(2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int. J. Pharm. 2000, 194, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Ashaduzzaman, M.; Mishra, P.; Swart, H.C.; Turner, A.P.F.; Tiwari, A. Stimuli-enabled zipper-like graphene interface for auto-switchable bioelectronics. Biosens. Bioelectron. 2017, 89, 305–311. [Google Scholar] [CrossRef]
- Wang, L.N.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Marken, F. The immobilisation and reactivity of Fe(CN)63−/4− in an intrinsically microporous polyamine (PIM-EA-TB). J. Solid State Electr. 2020, 24, 2797–2806. [Google Scholar] [CrossRef] [Green Version]
- Naegeli, R.; Redepenning, J.; Anson, F.C. Influence of supporting electrolyte concentration and composition on formal potentials and entropies of redox couples incorporated in Nafion coatings on electrodes. J. Phys. Chem. 1986, 90, 6227–6232. [Google Scholar] [CrossRef]
- Ugo, P.; Anson, F.C. Poly(2-Vinylpyrazine) as a Soluble Polymeric Ligand and as an Electrode Coating—Reactions with Pentacyanoferrate(Ii). Anal. Chem. 1989, 61, 1799–1805. [Google Scholar] [CrossRef]
- Doblhofer, K.; Vorotyntsev, M. ElectroactiVe Polymer Electrochemistry. In Part 1. Fundamentals; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Asturias, G.E.; Jang, G.W.; Macdiarmid, A.G.; Doblhofer, K.; Zhong, C.J. Membrane-Properties of Polymer-Films—The Acid-Doping Reaction of Polyaniline. Ber. Bunsen. Phys. Chem. 1991, 95, 1381–1384. [Google Scholar] [CrossRef]
- Schäfer, J.; Foest, R.; Ohl, A.; Weltmann, K.D. Miniaturized non-thermal atmospheric pressure plasma jet—Characterization of self-organized regimes. Plasma Phys. Control. Fusion 2009, 51, 124045. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. J. 2012, 10, 181–188. [Google Scholar] [CrossRef]










| n-HD Nebulizer Method (µm) | d-HD Droplet Method (µm) | |
|---|---|---|
| HD14 | 0.1 ± 0.00 | 0.5 ± 0.03 |
| HD11 | 0.4 ± 0.06 | 1.1 ± 0.16 |
| HD41 | 0.6 ± 0.02 | 1.5 ± 0.14 |
| d-HD14 | d-HD11 | d-HD41 | n-HD14 | n-HD11 | n-HD41 | |
|---|---|---|---|---|---|---|
| pH 4 | 36° ± 5° | 42° ± 5° | 43° ± 5° | 27° ± 6° | 28° ± 3° | 39° ± 5° |
| pH 7 | 43° ± 5° | 53° ± 1° | 56° ± 2° | 28° ± 6° | 34° ± 2° | 54° ± 2° |
| pH 10 | 77° ± 5° | 68° ± 4° | 63° ± 3° | 42° ± 8° | 41° ± 6° | 53° ± 4° |
| Air | pH 4 | pH 7 | pH 10 | |
|---|---|---|---|---|
| pp d-HD14 (µm) | 0.67 ± 0.05 | 1.08 ± 0.21 | 0.65 ± 0.02 | 0.83 ± 0.04 |
| pp d-HD11 (µm) | 0.69 ± 0.05 | 0.73 ± 0.05 | 0.78 ± 0.05 | 1.25 ± 0.20 |
| pp d-HD41 (µm) | 0.68 ± 0.02 | 0.74 ± 0.02 | 0.79 ± 0.09 | 1.36 ± 0.24 |
| Bare gold EL (µm) | 0.89 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levien, M.; Nasri, Z.; Weltmann, K.-D.; Fricke, K. Study on the Interaction of Plasma-Polymerized Hydrogel Coatings with Aqueous Solutions of Different pH. Gels 2023, 9, 237. https://doi.org/10.3390/gels9030237
Levien M, Nasri Z, Weltmann K-D, Fricke K. Study on the Interaction of Plasma-Polymerized Hydrogel Coatings with Aqueous Solutions of Different pH. Gels. 2023; 9(3):237. https://doi.org/10.3390/gels9030237
Chicago/Turabian StyleLevien, Monique, Zahra Nasri, Klaus-Dieter Weltmann, and Katja Fricke. 2023. "Study on the Interaction of Plasma-Polymerized Hydrogel Coatings with Aqueous Solutions of Different pH" Gels 9, no. 3: 237. https://doi.org/10.3390/gels9030237
APA StyleLevien, M., Nasri, Z., Weltmann, K. -D., & Fricke, K. (2023). Study on the Interaction of Plasma-Polymerized Hydrogel Coatings with Aqueous Solutions of Different pH. Gels, 9(3), 237. https://doi.org/10.3390/gels9030237