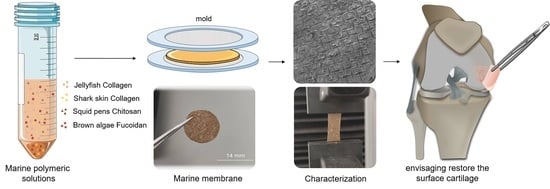
Advanced Polymeric Membranes as Biomaterials Based on Marine Sources Envisaging the Regeneration of Human Tissues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Green Metrics on Scaffolding Membrane Process
2.2. Determination of Glycosylation by Glycoprotein/Carbohydrate Estimation in Collagen Samples
2.3. 1H Nuclear Magnetic Resonance (1H-NMR) Analysis
2.4. Chemical Characterization of Polymeric Membranes
2.5. Physical Characterization of Polymeric Membranes
2.6. Thermal Characterization of Polymeric Membranes
2.7. Mechanical Tests by Tensile Strength
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Solutions and Marine Membranes Preparation
4.3. Marine Biopolymers and Membranes Characterization
4.3.1. Determination of Glycosylation by Glycoprotein/Carbohydrate Estimation in Collagen Samples
4.3.2. Quantification of Total Collagen Concentration
4.3.3. H Nuclear Magnetic Resonance (1H-NMR) Analysis
4.3.4. Ellman’s Test—Thiol Groups’ Quantification
4.3.5. Water Contact Angle Analysis
4.3.6. Surface and Depth Profile Analysis by X-ray Photoelectron Spectroscopy (XPS)
4.3.7. Scanning Electron Microscopy (SEM)
4.3.8. Surface Zeta (ζ) Potential Measurements
4.3.9. Swelling—Water Uptake Quantification
4.3.10. Differential Scanning Calorimetry (DSC)
4.3.11. Thermogravimetric Analysis (TGA)
4.3.12. Mechanical Tests by Tensile Strength
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Bo, R.; Zhang, Y. Polymeric scaffolds for regeneration of central/peripheral nerves and soft connective tissues. Adv. NanoBiomed Res. 2023, 3, 2200147. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Responte, D.J.; Natoli, R.M.; Athanasiou, K.A. Collagens of articular cartilage: Structure, function, and importance in tissue engineering. Cllinical Rev. Biomed. Eng. 2007, 35, 361–411. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gonçalves, C.; Oliveira, J.M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Innovative methodology for marine collagen–chitosan–fucoidan hydrogels production, tailoring rheological properties towards biomedical application. Green Chem. 2021, 23, 7016–7029. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Lopez-Cebral, R.; Sousa, R.O.; Alves, A.L.; Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Reis, R.L.; Silva, T.H. Marine collagen-chitosan-fucoidan cryogels as cell-laden biocomposites envisaging tissue engineering. Biomed. Mater. 2020, 15, 055030. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Cocce, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Perez-Martin, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-laden biomimetically mineralized shark-skin-collagen-based 3D printed hydrogels for the engineering of hard tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Reis, R.; Silva, T.H. Marine origin materials on biomaterials and advanced therapies to cartilage tissue engineering and regenerative medicine. Biomater. Sci. 2021, 9, 6718–6736. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Reis, R.L. Biopolymer membranes in tissue engineering. In Biopolymer Membranes and Films; De Moraes, M., Da Silva, C., Vieira, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–163. [Google Scholar]
- Rodrigues, M.N.; Oliveira, M.B.; Costa, R.R.; Mano, J.F. Chitosan/chondroitin sulfate membranes produced by polyelectrolyte complexation for cartilage engineering. Biomacromolecules 2016, 17, 2178–2188. [Google Scholar] [CrossRef]
- Cleymand, F.; Zhang, H.; Dostert, G.; Menu, P.; Arab-Tehrany, E.; Velot, E.; Mano, J.F. Membranes combining chitosan and natural-origin nanoliposomes for tissue engineering. RSC Adv. 2016, 6, 83626–83637. [Google Scholar] [CrossRef]
- He, X.; Fu, W.; Feng, B.; Wang, H.; Liu, Z.; Yin, M.; Wang, W.; Zheng, J. Electrospun collagen–poly(l-lactic acid-co-ecaprolactone) membranes for cartilage tissue engineering. Regen. Med. 2013, 8, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Inci, I.; Norouz Dizaji, A.; Ozel, C.; Morali, U.; Dogan Guzel, F.; Avci, H. Decellularized inner body membranes for tissue engineering: A review. J. Biomater. Sci. Polym. Ed. 2020, 31, 1287–1368. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.R.; Costa, A.M.S.; Caridade, S.G.; Mano, J.F. Compact saloplastic membranes of natural polysaccharides for soft tissue engineering. Chem. Mater. 2015, 27, 7490–7502. [Google Scholar] [CrossRef]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.; MoreiraTeixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.; Karperien, M.; Mano, J. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng. Part C Methods 2011, 22, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Reys, L.L.; Silva, S.S.; Oliveira, J.M.; Caridade, S.G.; Mano, J.F.; Silva, T.H.; Reis, R.L. Revealing the potential of squid chitosan-based structures for biomedical applications. Biomed. Mater. 2013, 8, 045002. [Google Scholar] [CrossRef]
- Martins, A.; Silva, M.A.; Costa Pinto, A.; Reis, R.L.; Neves, N.M. Bio-Inspired integration of natural materials. In Bio-Inspired Materials for Biomedical Engineering, 1st ed.; Brennan, A.B., Kirschner, C.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 125–150. [Google Scholar]
- Carretero, A.; Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Extracellular matrix-inspired assembly of glycosaminoglycan–collagen fibers. J. Mater. Chem. B 2017, 5, 3103–3106. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.; Marques, A.; Martins, E.; Silva, T.; Reis, R. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. Catalysis: The key to waste minimization. J. Chem. Technol. Biotechnol. 1997, 68, 381–388. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor at 30: A passion for pollution prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Garcia-Quintero, A.; Palencia, M. A critical analysis of environmental sustainability metrics applied to green synthesis of nanomaterials and the assessment of environmental risks associated with the nanotechnology. Sci. Total Environ. 2021, 793, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Morsali, M.; Liu, J.; Sipponen, M.H. Access to tough and transparent nanocomposites via Pickering emulsion polymerization using biocatalytic hybrid lignin nanoparticles as functional surfactants. Green Chem. 2021, 23, 3001–3014. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sricholpech, M.; Terajima, M.; Tomer, K.B.; Perdivara, I. Glycosylation of type I collagen. In Post-Translational Modification of Proteins: Tools for Functional Proteomics, Methods in Molecular Biology, 2019/07/01 ed; Kannicht, C., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2019; Volume 1934, pp. 127–144. [Google Scholar]
- Bann, J.G.; Bachinger, H.P. Glycosylation/Hydroxylation-induced stabilization of the collagen triple helix. 4-trans-hydroxyproline in the Xaa position can stabilize the triple helix. J. Biol. Chem. 2000, 275, 24466–24469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merly, L.; Smith, S.L. Collagen type II, alpha 1 protein: A bioactive component of shark cartilage. Int. Immunopharmacol. 2013, 15, 309–315. [Google Scholar] [CrossRef]
- Hennet, T. Collagen glycosylation. Curr. Opin. Struct. Biol. 2019, 56, 131–138. [Google Scholar] [CrossRef]
- Krishnamoorthi, J.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Isolation and partial characterization of collagen from outer skin of Sepia pharaonis (Ehrenberg, 1831) from Puducherry coast. Biochem. Biophys. Rep. 2017, 10, 39–45. [Google Scholar] [CrossRef]
- Fullerton, G.D.; Nes, E.; Amurao, M.; Rahal, A.; Krasnosselskaia, L.; Cameron, I. An NMR method to characterize multiple water compartments on mammalian collagen. Cell Biol. Int. 2006, 30, 66–73. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Won, H.-S.; Kim, W.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Isolation and characterisation of acid- and pepsin-soluble collagen from the skin of Cervus korean TEMMINCK var. mantchuricus Swinhoe. Anim. Prod. Sci. 2018, 58, 585–594. [Google Scholar] [CrossRef]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L.; Fanizzi, F.P. 1H NMR metabolic profile of Scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in Female Gonads and Somatic Tissues: Preliminary Results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef] [Green Version]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Zhao, Y. Characteristics of deacetylation and depolymerization of beta-chitin from jumbo squid (Dosidicus gigas) pens. Carbohydr. Res. 2011, 346, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Reys, L.L.; Silva, S.S.; Pirraco, R.P.; Marques, A.P.; Mano, J.F.; Silva, T.H.; Reis, R.L. Influence of freezing temperature and deacetylation degree on the performance of freeze-dried chitosan scaffolds towards cartilage tissue engineering. Eur. Polym. J. 2017, 95, 232–240. [Google Scholar] [CrossRef]
- López-Cebral, R.; Silva, T.H.; Oliveira, J.M.; Novoa Carballal, R.; Reis, R.L. High Molecular Weight Chitosan, Process for Obtaining and Uses Thereof—Association for the Advancement of Tissues Engineering and Cell Based Technologies & Therapies (A4TEC). PCT Patent WO/2019/064231, 2018. [Google Scholar]
- Czechowska-biskup, R.; Jarosinska, D.; Rokita, B.; Ulanski, P.; Rosiak, J. Determination of degree of deacetylation of chitosan—Comparison of methods. Prog. Chem. Appl. Chitin 2012, 17, 5–20. [Google Scholar]
- Hsu, S.; Whu, S.W.; Tsai, C.; Wu, Y.; Chen, H.; Hsieh, K. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef]
- Shiroma, R.; Uechi, T.K.S.; TaKo, M. Structural study of fucoidan from the brown seaweed Hizikia fusiformis. Food Sci. Technol. Res. 2008, 10, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Sudharsan, S.; Subhapradha, N.; Seedevi, P.; Shanmugam, V.; Madeswaran, P.; Shanmugam, A.; Srinivasan, A. Antioxidant and anticoagulant activity of sulfated polysaccharide from Gracilaria debilis (Forsskal). Int. J. Biol. Macromol. 2015, 81, 1031–1038. [Google Scholar] [CrossRef]
- Vasantharaja, R.; Stanley Abraham, L.; Jyotsna, J.; Seedevi, P.; Sathishkannan, G.; Thirugnanasambandam, R.; Kirubagaran, R. Sulfated polysaccharide from Sargassum tenerrimum attenuates oxidative stress induced reactive oxygen species production in in vitro and in zebrafish model. Carbohydr. Polym. 2019, 203, 441–449. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef] [Green Version]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; McDowell, R.A.; Shashkov, A.S.; Khatuntseva, E.A.; Usov, A.I.; Nifant’ev, N.E.; Haslam, S.M. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon-Lopez, A.; Fuentes-Jimenez, L.; Hernandez-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Alvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sawicki, L.A.; Kloxin, A.M. Design of thiol-ene photoclick hydrogels using facile techniques for cell culture applications. Biomater. Sci. 2014, 2, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, D.N.; Inácio, A.R.; Sousa, R.O.; Reis, R.L.; Silva, T.H. Seaweed polysaccharides as sustainable building blocks for biomaterials in tissue engineering. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 543–587. [Google Scholar]
- Santos, C.; Silva, C.J.; Büttelb, Z.; Guimarães, R.; Pereira, S.B.; Tamagnini, P.; Zille, A. Preparation and characterization of polysaccharides/PVA blend nanofibrous membranes by electrospinning method. Carbohydr. Polym. 2014, 99, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.J.; Goncalves, C.P.; Galvao, K.M.; D’Alpino, P.H.P.; Nascimento, F.D. Synthesis and characterizations of a collagen-rich biomembrane with potential for tissue-guided regeneration. Eur. J. Dent. 2019, 13, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edalatpoura, M.; Liu, L.; Jacobi, A.M.; Eid, K.F.; Sommersa, A.D. Managing water on heat transfer surfaces: A critical review of techniques to modify surface wettability for applications with condensation or evaporation. Appl. Energy 2018, 222, 967–992. [Google Scholar] [CrossRef]
- Agrawal, D.C. (Ed.) Surfaces. In Introduction to Nanoscience and Nanomaterials; Springer Nature: Berlin/Heidelberg, Germany, 2013; Volume 1142/8433, pp. 9–64. [Google Scholar]
- Jeuken, R.M.; Roth, A.K.; Peters, R.; Van Donkelaar, C.C.; Thies, J.C.; Van Rhijn, L.W.; Emans, P.J. Polymers in cartilage defect repair of the knee: Current status and future prospects. Polymers 2016, 8, 219. [Google Scholar] [CrossRef]
- Agger, J.W.; Nilsen, P.J.; Eijsink, V.G.H.; Horn, S.J. On the determination of water content in biomass processing. BioEnergy Res. 2013, 7, 442–449. [Google Scholar] [CrossRef]
- Isengard, H. Water content, one of the most important properties of food. Food Control 2001, 12, 395–400. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; IntechOpen: London, UK, 2011; pp. 117–150. [Google Scholar]
- León-Mancilla, B.H.; Araiza-Téllez, M.A.; Flores-Flores, J.O.; Piña-Barba, M.C. Physico-chemical characterization of collagen scaffolds for tissue engineering. J. Appl. Res. Technol. 2016, 14, 77–85. [Google Scholar] [CrossRef]
- Talik, P.; Hubicka, U. The DSC approach to study non-freezing water contents of hydrated hydroxypropylcellulose (HPC). J. Therm. Anal. Calorim. 2017, 132, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Pace, C.N.; Fu, H.; Lee Fryar, K.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Zee, M.Y.; Sobernheim, D.; Garzon, J.R. Glossary for unconventional oil and gas resource evaluation and development. In Unconventional Oil and Gas Resources Handbook; Zee, M.Y., Holditch, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 513–526. [Google Scholar]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ateshian, G.A.; Warden, W.H.; Kim, J.J.; Grelsamer, R.P.; Mow, V.C. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. J. Biommechanics 1997, 30, 1157–1164. [Google Scholar] [CrossRef]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the mechanical environment on the regeneration of osteochondral defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef]
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A review of mechanical properties of scaffold in tissue engineering: Aloe Vera composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Williams, D.S.; Sotelo, C.G.; Pérez-Martín, R.I.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. Marine origin biomaterials using a compressive and absorption methodology as cell-laden hydrogel envisaging cartilage tissue engineering. Biomater. Adv. 2022, 137, 212843. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.N.; Goncalves, C.; Oliveira, J.M.; Williams, D.S.; Mearns-Spragg, A.; Reis, R.L.; Silva, T.H. A design of experiments (DoE) approach to optimize cryogel manufacturing for tissue engineering applications. Polymers 2022, 14, 2026. [Google Scholar] [CrossRef] [PubMed]
- Diogo, G.S.; Senra, E.L.; Pirraco, R.P.; Canadas, R.F.; Fernandes, E.M.; Serra, J.; Perez-Martin, R.I.; Sotelo, C.G.; Marques, A.P.; Gonzalez, P.; et al. Marine collagen/apatite composite scaffolds envisaging hard tissue applications. Mar. Drugs 2018, 16, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehy, E.J.; Lemoine, M.; Clarke, D.; Vazquez, A.G.; O’Brien, F.J. The incorporation of marine coral microparticles into collagen-based scaffolds promotes osteogenesis of human mesenchymal stromal cells via calcium ion signalling. Mar. Drugs 2020, 18, 74. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Fu, C.; Wu, S.; Liu, G.; Guo, J.; Su, Z.J.M.d. Determination of the deacetylation degree of chitooligosaccharides. Mar. Drugs 2017, 15, 332. [Google Scholar] [CrossRef]
- Hauptstein, S.; Muller, C.; Dunnhaupt, S.; Laffleur, F.; Bernkop-Schnurch, A. Preactivated thiomers: Evaluation of gastroretentive minitablets. Int. J. Pharm. 2013, 456, 473–479. [Google Scholar] [CrossRef]
- Huamani-Palomino, R.G.; Cordova, B.M.; Pichilingue, L.E.; Venancio, T.; Valderrama, A.C. Functionalization of an alginate-based material by oxidation and reductive amination. Polymers 2021, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.; Seah, M. XPS—Practical Surface Analysis, 2nd ed.; Sons, J.W., Ed.; Wiley: Chichester, UK, 1993; Volume 1. [Google Scholar]
- Zayed, A.; Hahn, T.; Finkelmeier, D.; Burger-Kentischer, A.; Rupp, S.; Krämer, R.; Ulber, R. Phenomenological investigation of the cytotoxic activity of fucoidan isolated from Fucus vesiculosus. Process Biochem. 2019, 81, 182–187. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from Undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.S.; Ulber, R. Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef] [PubMed]












| Samples | |||
|---|---|---|---|
| jCOL | - | - | 0.148 ± 0.030 |
| sCOL | - | - | 0.234 ± 0.010 |
| sCHT | 0.005 ± 0.000 | 0.050 ± 0.000 | 0.102 ± 0.014 |
| aFUC | 0.029 ± 0.000 | 2.101 ± 0.084 | 0.014 ± 0.000 |
| M/J3 | 0.013 ± 0.001 | 0.325 ± 0.091 | 0.041 ± 0.006 |
| M/J5 | 0.021 ± 0.007 | 0.341 ± 0.137 | 0.063 ± 0.003 |
| M/S3 | 0.017 ± 0.005 | 0.339 ± 0.019 | 0.050 ± 0.020 |
| M/S5 | 0.024 ± 0.001 | 0.585 ± 0.030 | 0.041 ± 0.004 |
| M/J3S3 | 0.018 ± 0.001 | 0.356 ± 0.024 | 0.051 ± 0.003 |
| M/J5S3 | 0.016 ± 0.000 | 0.302 ± 0.020 | 0.053 ± 0.001 |
| M/J3S5 | 0.016 ± 0.000 | 0.220 ± 0.011 | 0.075 ± 0.004 |
| M/J5S5 | 0.014 ± 0.001 | 0.265 ± 0.007 | 0.054 ± 0.006 |
| Polymeric Membrane Systems (100%) | Abbreviation | mg/mL of Polymer in the Original Solution | |||
|---|---|---|---|---|---|
| Collagen Jellyfish | Collagen Shark | Chitosan Squid Pens | Fucoidan Seaweed | ||
| jCOL3/sCHT/aFUC | M/J3 | 30; (18.8) | -; (0) | 30; (18.8) | 100; (62.4) |
| jCOL5/sCHT/aFUC | M/J5 | 50; (27.8) | -; (0) | 30; (16.6) | 100; (55.6) |
| sCOL3/sCHT/aFUC | M/S3 | -; (0) | 30; (18.8) | 30; (18.8) | 100; (62.4) |
| sCOL5/sCHT/aFUC | M/S5 | -; (0) | 50; (27.8) | 30; (16.6) | 100; (55.6) |
| jCOL3/sCOL3/sCHT/aFUC | M/J3S3 | 30; (15.8) | 30; (15.8) | 30; (15.8) | 100; (52.6) |
| jCOL5/sCOL3/sCHT/aFUC | M/J5S3 | 50; (23.8) | 30; (14.3) | 30; (14.3) | 100; (47.6) |
| jCOL3/sCOL5/sCHT/aFUC | M/J3S5 | 30; (14.3) | 50; (23.8) | 30; (14.3) | 100; (47.6) |
| jCOL5/sCOL5/sCHT/aFUC | M/J5S5 | 50; (21.7) | 50; (21.7) | 30; (13.1) | 100; (43.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.N.; Lobo, F.C.M.; Rodrigues, L.C.; Fernandes, E.M.; Williams, D.S.; Mearns-Spragg, A.; Sotelo, C.G.; Perez-Martín, R.I.; Reis, R.L.; Gelinsky, M.; et al. Advanced Polymeric Membranes as Biomaterials Based on Marine Sources Envisaging the Regeneration of Human Tissues. Gels 2023, 9, 247. https://doi.org/10.3390/gels9030247
Carvalho DN, Lobo FCM, Rodrigues LC, Fernandes EM, Williams DS, Mearns-Spragg A, Sotelo CG, Perez-Martín RI, Reis RL, Gelinsky M, et al. Advanced Polymeric Membranes as Biomaterials Based on Marine Sources Envisaging the Regeneration of Human Tissues. Gels. 2023; 9(3):247. https://doi.org/10.3390/gels9030247
Chicago/Turabian StyleCarvalho, Duarte Nuno, Flávia C. M. Lobo, Luísa C. Rodrigues, Emanuel M. Fernandes, David S. Williams, Andrew Mearns-Spragg, Carmen G. Sotelo, Ricardo I. Perez-Martín, Rui L. Reis, Michael Gelinsky, and et al. 2023. "Advanced Polymeric Membranes as Biomaterials Based on Marine Sources Envisaging the Regeneration of Human Tissues" Gels 9, no. 3: 247. https://doi.org/10.3390/gels9030247
APA StyleCarvalho, D. N., Lobo, F. C. M., Rodrigues, L. C., Fernandes, E. M., Williams, D. S., Mearns-Spragg, A., Sotelo, C. G., Perez-Martín, R. I., Reis, R. L., Gelinsky, M., & Silva, T. H. (2023). Advanced Polymeric Membranes as Biomaterials Based on Marine Sources Envisaging the Regeneration of Human Tissues. Gels, 9(3), 247. https://doi.org/10.3390/gels9030247