Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study
Abstract
:1. Introduction
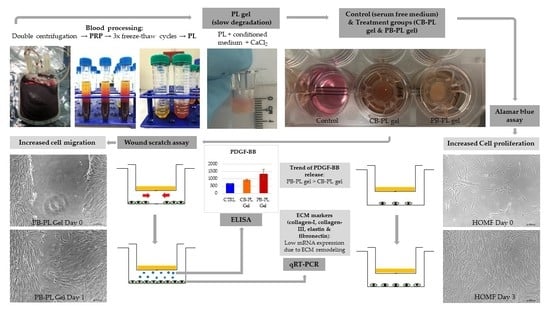
2. Results and Discussion
2.1. Cell Proliferation in PL Gels
2.2. Cell Migration in Wound Healing
2.3. Gel Degradation
2.4. ECM Gene Expression in Wound Remodeling
2.5. PDGF-BB Released from PL Gels
2.6. Limitation of Study and Future Studies
3. Conclusions
4. Materials and Methods
4.1. Tissue Collection and Handling
4.1.1. Cord Blood Collection and Processing
4.1.2. Peripheral Blood Collection and Processing
4.1.3. Isolation and Culture of Primary Human Oral Mucosal Fibroblasts
4.1.4. Platelet Lysate Gel Preparation
4.2. Alamar Blue Cell Proliferation Assay
4.3. Wound Scratch Assay
4.4. Gel Degradation Measurement
4.5. Two-Step Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-qPCR)
4.6. PDGF-BB Measurement via Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakiawati, D.; Nur’aeny, N.; Setiadhi, R. Distribution of oral ulceration cases in oral medicine integrated installation of Universitas padjadjaran dental hospital. Padjadjaran J. Dent. 2020, 32, 237–242. [Google Scholar] [CrossRef]
- Fitzpatrick, S.G.; Cohen, D.M.; Clark, A.N. Ulcerated Lesions of the Oral Mucosa: Clinical and Histologic Review. Head Neck Pathol. 2019, 13, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, F.; Casciaro, B.; Mangoni, M.L. A Novel In Vitro Wound Healing Assay to Evaluate Cell Migration. J. Vis. Exp. 2018, 17, e56825. [Google Scholar]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef]
- Wang, P.; Hu, Z.; Cao, X.; Huang, S.; Dong, Y.; Cheng, P.; Xu, H.; Shu, B.; Xie, J.; Wu, J.; et al. Therapy, Fibronectin precoating wound bed enhances the therapeutic effects of autologous epidermal basal cell suspension for full-thickness wounds by improving epidermal stem cells’ utilization. Stem Cell Res. Ther. 2019, 10, 154. [Google Scholar] [CrossRef]
- Sarangthem, V.; Singh, T.D.; Dinda, A.K. Emerging Role of Elastin-Like Polypeptides in Regenerative Medicine. Adv. Wound Care 2021, 10, 257–269. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; Garcia-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Orlando, N.; Pellegrino, C.; Valentini, C.G.; Bianchi, M.; Barbagallo, O.; Sparnacci, S.; Forni, F.; Fontana, T.M.; Teofili, L. Umbilical cord blood: Current uses for transfusion and regenerative medicine. Transfus. Apher. Sci. 2020, 59, 102952. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumaran, M.S. Platelet-rich plasma: The journey so far! Indian Dermatol. Online J. 2020, 11, 685. [Google Scholar]
- Laner-Plamberger, S.; Oeller, M.; Mrazek, C.; Hartl, A.; Sonderegger, A.; Rohde, E.; Strunk, D.; Schallmoser, K. Upregulation of mitotic bookmarking factors during enhanced proliferation of human stromal cells in human platelet lysate. J. Transl. Med. 2019, 17, 432. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Wijaya, B.; Mobus, L.; Rademacher, F.; Rodewald, M.; Tohidnezhad, M.; Pufe, T.; Drucke, D.; Glaser, R.; Harder, J. Platelet-Released Growth Factors and Platelet-Rich Fibrin Induce Expression of Factors Involved in Extracellular Matrix Organization in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 4404. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Law, J.X.; Ng, S.-F. Delivery systems for platelet derived growth factors in wound healing: A review of recent developments and global patent landscape. J. Drug Deliv. Sci. Technol. 2022, 71, 103270. [Google Scholar] [CrossRef]
- Losi, P.; Barsotti, M.C.; Foffa, I.; Buscemi, M.; De Almeida, C.V.; Fabbri, M.; Gabbriellini, S.; Nocchi, F.; Ursino, S.; Urciuoli, P.; et al. In vitro human cord blood platelet lysate characterisation with potential application in wound healing. Int. Wound J. 2020, 17, 65–72. [Google Scholar] [CrossRef]
- Valentini, C.G.; Nuzzolo, E.R.; Bianchi, M.; Orlando, N.; Iachininoto, M.G.; Pinci, P.; Teofili, L. Cord Blood Platelet Lysate: In Vitro Evaluation to Support the Use in Regenerative Medicine. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019021. [Google Scholar]
- Sindici, E.; Basiglio, L.; Cafaro, A.; Fazio, L.; Dragonetti, A.; Pugliese, M.; Carossa, S.; Broccoletti, R.; Arduino, P.G. The photobiomodulation therapy together with the use of cord blood platelet gel could be safely suggested as primary treatment for oral lesions in patients with inherited epidermolysis bullosa. Photodermatol. Photoimmunol. Photomed. 2020, 36, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.-S.; Mahmoodi, M.; Rafati, A.R.; Manafi, F.; Mehrabani, D. The role of human adult peripheral and umbilical cord blood platelet-rich plasma on proliferation and migration of human skin fibroblasts. World J. Plast. Surg. 2017, 6, 198–205. [Google Scholar]
- Bayer, A.; Wijaya, B.; Rademacher, F.; Mobus, L.; Preuss, M.; Singh, M.; Tohidnezhad, M.; Kubo, Y.; Rodewald, M.; Behrendt, P.; et al. Platelet-Released Growth Factors Induce Genes Involved in Extracellular Matrix Formation in Human Fibroblasts. Int. J. Mol. Sci. 2021, 22, 10536. [Google Scholar] [CrossRef]
- Parazzi, V.; Lavazza, C.; Boldrin, V.; Montelatici, E.; Pallotti, F.; Marconi, M.; Lazzari, L. Extensive Characterization of Platelet Gel Releasate From Cord Blood in Regenerative Medicine. Cell Transpl. 2015, 24, 2573–2584. [Google Scholar] [CrossRef]
- Shirzad, N.; Bordbar, S.; Goodarzi, A.; Mohammad, M.; Khosravani, P.; Sayahpour, F.; Eslaminejad, M.B.; Ebrahimi, M. Umbilical cord blood platelet lysate as serum substitute in expansion of human mesenchymal stem cells. Cell J. (Yakhteh) 2017, 19, 403. [Google Scholar]
- Scopelliti, F.; Cattani, C.; Dimartino, V.; Mirisola, C.; Cavani, A. Platelet Derivatives and the Immunomodulation of Wound Healing. Int. J. Mol. Sci. 2022, 23, 8370. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.B.; Park, G.S.; Park, S.S.; Jang, Y.J.; Kim, K.H.; Kim, K.J.; Park, E.J. Effect of platelet-rich plasma on proliferation and migration in human dermal fibroblasts. J. Cosmet. Dermatol. 2019, 18, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S.; Kim, S. An Insight into Recent Advances on Platelet Function in Health and Disease. Int. J. Mol. Sci. 2022, 23, 6022. [Google Scholar] [CrossRef]
- Troha, K.; Vozel, D.; Arko, M.; Bedina Zavec, A.; Dolinar, D.; Hočevar, M.; Jan, Z.; Kisovec, M.; Kocjančič, B.; Pađen, L.; et al. Autologous Platelet and Extracellular Vesicle-Rich Plasma as Therapeutic Fluid: A Review. Int. J. Mol. Sci. 2023, 24, 3420. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.P.B.; Domingues, R.M.A.; Shevchuk, M.; Gomes, M.E.; Peppas, N.A.; Reis, R.L. Biomaterials for Sequestration of Growth Factors and Modulation of Cell Behavior. Adv. Funct. Mater. 2020, 30, 1909011. [Google Scholar] [CrossRef]
- Sam, G.; Vadakkekuttical, R.J.; Amol, N.V. In vitro evaluation of mechanical properties of platelet-rich fibrin membrane and scanning electron microscopic examination of its surface characteristics. J. Indian Soc. Periodontol. 2015, 19, 32–36. [Google Scholar] [CrossRef]
- Cavallo, C.; Roffi, A.; Grigolo, B.; Mariani, E.; Pratelli, L.; Merli, G.; Kon, E.; Marcacci, M.; Filardo, G. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. BioMed Res. Int. 2016, 2016, 6591717. [Google Scholar] [CrossRef]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. [Google Scholar] [CrossRef]
- Chatterjee, A.; Debnath, K. Comparative evaluation of growth factors from platelet concentrates: An in vitro study. J. Indian Soc. Periodontol. 2019, 23, 322–328. [Google Scholar]
- Hudgens, J.L.; Sugg, K.B.; Grekin, J.A.; Gumucio, J.P.; Bedi, A.; Mendias, C.L. Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts. Am. J. Sports Med. 2016, 44, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. Bras. Derm. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. 1), 177–183. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Toriseva, M.; Laato, M.; Carpen, O.; Ruohonen, S.T.; Savontaus, E.; Inada, M.; Krane, S.M.; Kahari, V.M. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLoS ONE 2012, 7, e42596. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.F.; Yahya, M.A.A.M.; Azhar, N.A.; Ibrahim, N.; Ghafar, N.A.; Ghani, N.A.A.; Nizar, M.A.M.; Yunus, S.S.M.; Singh, T.K.L.; Law, J.-X.; et al. In Vitro Cell Proliferation and Migration Properties of Oral Mucosal Fibroblasts: A Comparative Study on the Effects of Cord Blood-and Peripheral Blood-Platelet Lysate. Int. J. Mol. Sci. 2023, 24, 5775. [Google Scholar] [CrossRef]
- Hassan, M.N.F.; Yap, Z.Y.; Tang, Y.L.; Ng, M.H.; Law, J.X. Expired platelet concentrate as a source of human platelet lysate for Xenogeneic-free culture of human dermal fibroblasts. Sains Malays. 2021, 50, 2355–2365. [Google Scholar] [CrossRef]
- Noh, K.C.; Liu, X.N.; Zhuan, Z.; Yang, C.J.; Kim, Y.T.; Lee, G.W.; Choi, K.H.; Kim, K.O. Leukocyte-Poor Platelet-Rich Plasma-Derived Growth Factors Enhance Human Fibroblast Proliferation In Vitro. Clin. Orthop. Surg. 2018, 10, 240–247. [Google Scholar] [CrossRef]
- Mousavi, S.; Zarrabi, M.; Abroun, S.; Ahmadipanah, M.; Abbaspanah, B. Umbilical cord blood quality and quantity: Collection up to transplantation. Asian J. Transfus. Sci. 2019, 13, 79–89. [Google Scholar] [CrossRef]
- Bullard, J.D.; Lei, J.; Lim, J.J.; Massee, M.; Fallon, A.M.; Koob, T.J. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.J.; Chowdhury, S.R.; Bin Saim, A.; Idrus, R.B. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy 2015, 17, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.-R.; Sahardi, N.F.N.M.; Tan, J.; Chua, K.-H.; Wan, W.Z. Characterization of Keratinocytes, Fibroblasts and Melanocytes Isolated from Human Skin using Gene Markers. Sains Malays. 2022, 51, 1425–1436. [Google Scholar] [CrossRef]










| ECM Marker | Group | Relative mRNA Expression to GAPDH (Mean ± SEM) |
|---|---|---|
| Col. I | CTRL | 1.47 ± 0.35 |
| CB-PL gel | 0.13 ± 0.07 | |
| PB-PL gel | 0.09 ± 0.06 | |
| Col. III | CTRL | 0.64 ± 0.13 |
| CB-PL gel | 0.09 ± 0.03 | |
| PB-PL gel | 0.05 ± 0.02 | |
| Elastin | CTRL | 0.0135 ± 0.0042 |
| CB-PL gel | 0.0018 ± 0.0010 | |
| PB-PL gel | 0.0012 ± 0.0007 | |
| Fibronectin | CTRL | 2.28 ± 0.45 |
| CB-PL gel | 1.47 ± 0.35 | |
| PB-PL gel | 0.87 ± 0.22 |
| Gene | GenBank Accession Number | Primer Sequence (5′ to 3′) |
|---|---|---|
| GAPDH | NM_002046.5 | F: CAATGACCCCTTCATTGACC |
| R: TTGATTTTGGAGGGATCTCG | ||
| Collagen-I | NM_000088.3 | F: GTGCTAAAGGTGCCAATGGT |
| R: ACCAGGTTCACCGCTGTTAC | ||
| Collagen-III | NM_000090.3 | F: CCAGGAGCTAACGGTCTCAG |
| R: CAGGGTTTCCATCTCTTCCA | ||
| Elastin | NM_000501.4 | F: GGTGGCTTAGGAGTGTCTGC |
| R: CCAGCAAAAGCTCCACCTAC | ||
| Fibronectin | NM_212482.2 | F: AAAATGGCCAGATGATGAGC |
| R: TGGCACCGAGATATTCCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.-L.; Azhar, N.A.; Budin, S.B.; Ibrahim, N.; Abdul Ghani, N.A.; Abd Ghafar, N.; Law, J.-X. Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study. Gels 2023, 9, 343. https://doi.org/10.3390/gels9040343
Ng S-L, Azhar NA, Budin SB, Ibrahim N, Abdul Ghani NA, Abd Ghafar N, Law J-X. Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study. Gels. 2023; 9(4):343. https://doi.org/10.3390/gels9040343
Chicago/Turabian StyleNg, Sook-Luan, Nur Ain Azhar, Siti Balkis Budin, Norliwati Ibrahim, Nur Azurah Abdul Ghani, Norzana Abd Ghafar, and Jia-Xian Law. 2023. "Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study" Gels 9, no. 4: 343. https://doi.org/10.3390/gels9040343
APA StyleNg, S. -L., Azhar, N. A., Budin, S. B., Ibrahim, N., Abdul Ghani, N. A., Abd Ghafar, N., & Law, J. -X. (2023). Effects of Platelet Lysate Gels Derived from Different Blood Sources on Oral Mucosal Wound Healing: An In Vitro Study. Gels, 9(4), 343. https://doi.org/10.3390/gels9040343